An Introduction to Organic Compounds: Nomenclature,Physical
advertisement

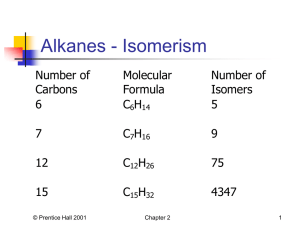
Contents Structure and Nomenclature of Simple Hydrocarbons Organic Compounds ( Alkanes , alkenes and their Cyclic Compounds ) Constitution – Configuration – Conformation 1 Alkanes: Nomenclature Organic nomenclature is based on alkanes: Contain only carbon and hydrogen (C, H). All carbon atoms are sp3 hybridized. © Prentice Hall 2001 Chapter 2 2 Alkanes - Isomerism Number of Carbons 6 Molecular Formula C6H14 Number of Isomers 5 7 C7H16 9 12 C12H26 75 15 C15H32 4347 © Prentice Hall 2001 Chapter 2 3 Nomenclature The International Union of Pure and Applied Chemistry (IUPAC) has established the nomenclature system. Systematic nomenclature (IUPAC nomenclature) (shown in blue). Additional names such as isopentane and neopentane are common names (dt.: Trivialnamen; shown in red). A compound may have more than one name but a name must specify only one compound. © Prentice Hall 2001 Chapter 2 4 Conformations of Alkanes: Rotation about C-C Single Bonds Different spatial arrangements of atoms that result from rotation about carbon-carbon single bonds are known as conformations. Different conformations also are called conformational isomers or conformers: © Prentice Hall 2001 Chapter 2 5 Newman Projections A convenient way to describe conformation isomers is to look at the molecule along the axis of the bond of interest. A Newman projection is a graphical representation of such a view: © Prentice Hall 2001 Chapter 2 6 Conformations of Alkanes: Rotation About C-C Single Bonds When ethane molecules rotate about the carbon-carbon single bond various isomers result, two of them are: staggered or anti conformation © Prentice Hall 2001 eclipsed or syn conformation. Chapter 2 7 Conformations of Alkanes: Rotation About C-C Single Bonds © Prentice Hall 2001 Chapter 2 8 Conformations of Alkanes: Rotation About C-C Single Bonds The extra energy of the eclipsed conformation is called torsional strain: © Prentice Hall 2001 Chapter 2 9 Conformations of Butane: Rotation About the C2-C3 Single Bond © Prentice Hall 2001 Chapter 2 10 Conformations of Butane: Rotation About the C2-C3 Single Bond © Prentice Hall 2001 Chapter 2 11 Nomenclature Typically, any unspecified alkyl group is represented as R© Prentice Hall 2001 Chapter 2 12 Nomenclature 1. Rule: Count the longest continuous straight chain to constitute a compound name: Note that these compounds all have the same sum formula C7H16; they are isomers. © Prentice Hall 2001 Chapter 2 13 Nomenclature of Alkyl Substituents The name of the alkyl group followed by the name of the class of compound constitutes the common name for alcohols amines, and alkyl halides: © Prentice Hall 2001 Chapter 2 14 Alkyl Nomenclature A primary carbon is one that is bonded to only one other carbon, frequently represented by 1 A primary hydrogen is attached to a primary carbon: © Prentice Hall 2001 Chapter 2 15 Alkyl Nomenclature A secondary carbon is one that is bonded to two other carbons, represented by 2, also by sec- or s- A secondary hydrogen is attached to a secondary carbon: © Prentice Hall 2001 Chapter 2 16 Alkyl Nomenclature A tertiary carbon is one that is bonded to three other carbons, represented by 3, also by tert- or t- A tertiary hydrogen is attached to a tertiary carbon: Reactivity often depends on whether a carbon or hydrogen is 1, 2, or 3! © Prentice Hall 2001 Chapter 2 17 Nomenclature of Alkyl Substituents There are four alkyl groups that contain four carbons: © Prentice Hall 2001 Chapter 2 18 Nomenclature of Alkyl Substituents © Prentice Hall 2001 Chapter 2 19 IUPAC Systematic Nomenclature: Alkanes 1. Determine longest continuous chain (i.e. parent hydrocarbon). 2. Cite the name of substituent before the name of the parent hydrocarbon along with the number of the carbon to which it is attached: © Prentice Hall 2001 Chapter 2 20 IUPAC Systematic Nomenclature: Alkanes 3. Number in the direction that gives the lower number for the lowest-numbered substituent. 4. Substituents are listed in alphabetical order – neglecting prefixes such as di- tri- tert- etc. © Prentice Hall 2001 Chapter 2 21 IUPAC Systematic Nomenclature: Alkanes 4. When both directions yield the same lower number for the lowest numbered substituent, select the direction that yields the lower number for the next lowest numbered substituent: 5. If same substituent numbers are obtained in either direction, number in direction giving lowest number to the first named substituent. © Prentice Hall 2001 Chapter 2 22 IUPAC Systematic Nomenclature: Alkanes 6. If compound has two or more chains of the same length, parent hydrocarbon is chain with higher number of substituents: © Prentice Hall 2001 Chapter 2 23 Nomenclature of Cycloalkanes Cycloalkanes generally are shown as skeletal structures: © Prentice Hall 2001 Chapter 2 24 Nomenclature of Cycloalkanes 1. Ring is the parent hydrocarbon unless the alkyl substituent has more carbons; in that case the substituent becomes the parent hydrocarbon. If only one substituent, no need to give it a number: © Prentice Hall 2001 Chapter 2 25 Nomenclature of Cycloalkanes 2. If the ring has 2 substituents, list in alphabetical order and give number 1 to first named group: © Prentice Hall 2001 Chapter 2 26