pola27755-sup-0001
advertisement

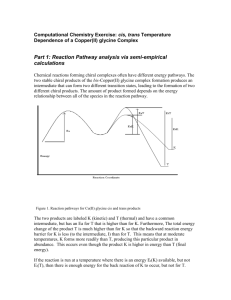
Supporting Information Photochemical Stability of Dicyano-Substituted Poly(phenylenevinylenes) with Different Side Chains Logan P. Sanow, Jianyuan Sun, Cheng Zhang* Department of Chemistry and Biochemistry South Dakota State University, Brookings, South Dakota 57006 TABLE OF CONTENTS Page IR spectra of RO-diCN-PPV worked up in MeOH and dilute HCl S2 1 S2-S3 H-NMR spectra of RO-diCN-PPV and C8-diCN-PPV IR of RO-diCN-PPV doped with carboxylic acid, phenol, and alkyl alcohol S3 Light-induced change in polymer molecular weight and GPC elugram profiles S4-7 C8-diCN-PPV phosphorescence lifetimes S8 PL spectra of C8-DiCN-PPV and RO-DiCN-PPV S9 PL spectra of RO-diCN-PPV and RO-diCN-PPV after illumination S9-10 S1 0.14 0.12 0.08 0.06 absorbance 0.10 0.04 0.02 0.00 4000 3500 3000 2500 2000 wavenumber (cm-1) 1500 1000 500 Figure 1S. The IR spectra of RO-diCN-PPV worked up in MeOH (blue) and worked up with dilute HCl (red). When the polymers were worked up by a direct precipitation of the basic reaction mixture in MeOH, the intermediate did not have a chance to be hydrolyzed into the imide, as a result, an imide peak (1710 cm-1) is not seen in the IR (blue). 1.2 1 0 0.8 -1 0.6 -2 0.4 TGA 0.2 μV (eotherm up) normalized weight 1 -3 DTA 0 -4 25 125 225 325 Temperature (C°) 425 Figure 2S. Thermal analysis of C8-diCN-PPV. Scan rate: 10 oC/min. N2. (We could obtain flat baseline for DTA with the Seiko DTA/TGA system) S2 Logan.396.esp 0.065 0.060 0.055 0.050 Normalized Intensity 0.045 0.040 0.035 0.030 0.025 0.020 0.015 trans cis 0.010 0.005 8 7 6 Logan.397.esp 5 4 Chemical Shift (ppm) 3 2 1 0.040 0.035 Normalized Intensity 0.030 0.025 0.020 0.015 0.010 trans cis 0.005 8 7 6 5 4 Chemical Shift (ppm) 3 2 Figure 3S. 1H-NMR spectra of RO-diCN-PPV (top) and C8-diCN-PPV in CDCl3. S3 1 p-methoxybenzoic acid 0.39 p-methoxy phenol 2-hexyl-1-decanol 0.19 absorbance 0.29 0.09 4000 3500 3000 2500 2000 wavenumber (cm -1) 1500 1000 500 -0.01 Figure 4S. IR spectra of RO-diCN-PPV with 15% p-methoxybenzoic acid (blue), 15% p-methoxy phenol (red), and 15% of 2-hexyl-1-decanol (green). Time (minutes) 0 50 100 150 200 250 0 Log (normalized absorbance) -0.2 -0.4 -0.6 -0.8 -1 -1.2 -1.4 -1.6 Figure 5S. Plot of the C8-diCN-PPV carbonyl peak height at 1709 cm-1 (blue) and the OH peak height at 3370 cm-1 (green) versus (accumulated) film illumination time. Light-induced change in polymer molecular weight and GPC elugram profiles S4 GPC elugrams of both polymers of different illumination time (0, 60, 120, 180 minutes) are shown in Figure 6S. Shift in peaks to longer elution time (lower MW) and growth of monomer/oligomer peaks are seen for both polymers, but more pronounced for C8-diCN-PPV, in agreement with the faster decomposition of this polymer. It is also noticed that the two polymers displaced different (opposite) changes in their GPC elugram profile with illumination. C8-diCN-PPV showed a symmetric peak before illumination. After 60 minutes of illumination, a high MW shoulder peak appeared and the long MW tail shifted to left and became stronger, while the main peak shifted to the right (lower MW). On contrary, RO-diCN-PPV showed a clear high MW shoulder before illumination, which disappeared after 60 minutes of illumination. 0 min 60 min 120 min 180 min 0.5 normalized absorbance normalized absorbance 0.6 0.5 0.4 0.3 0.2 0.4 0 60 0.3 120 180 0.2 0.1 0.1 0 0 12 14 16 18 11 20 Elution time (min) 13 15 17 Elution time (min) 19 FIGURE 6S. GPC elugrams for C8-diCN-PPV (Top) and RO-diCN-PPV (bottom) at different total illumination times. The UV-vis detector was set at 310 nm. The integration of absorbance in the displayed range is normalized for every elugram. To gain insight into the different behavior, GPC elugrams and 1H NMR spectra were recorded for both polymers at the early stage of illumination (0, 1, 2, … minutes) to show the evolution of GPC profiles and to find whether cis/trans content and photoinduced cis-trans isomerization are responsible for the change. Figure 7S left shows that the new GPC profile of C8-diCN-PPV was established after only two minutes of illumination, in coincidence with the time taken for this polymer to complete light-induced cistrans isomerization (Figure 8S left). So, the profile change is mostly likely due to the isomerization. However, if such change happened uniformly to all polymer chains, only a shift of the whole peak to the left, and no shoulder, should be observed. Since the vinylene units in C8-diCN-PPV are about half cis and half trans (Figure 8S left), low oligomers containing trans vinylene units (hereafter referred to as trans oligomers) are mainly responsible for light absorption of this polymer as cis is an less efficient conjugation unit and is also less planar due to higher steric hindrance (Figure 9S). Polymer chains that contain longer trans oligomers function as energy traps and get most of the light energy absorbed by the polymer film and cis-to-trans transformation mainly happen to these polymer chains, leading to a shoulder peak at the high MW side. In RO-diCN-PPV, trans dominates (likely as a result of significantly lower energy of trans as compared to cis), and the polymer has larger MW, so absorption wavelength of all polymer chains are similar (i.e., not S5 dependent on polymer length) and therefore, all polymer chains underwent cis- trans isomerization and experienced an increase in trans content. As a result, the retention time decreased from 14.925 min for the pristine polymer to 14.876 min after illumination of 1 min. As it only took 10 minutes for isomerization to reach equilibrium (Figure 8S right), cis-to-trans conversion rate decreased fast. From 2 min on, retention time started to increase (slowly), suggesting that photodegradation started to dominate MW change. More notably, the high MW shoulder peak weakened along with illumination and disappeared at 4 min. Presumably, the shoulder can be attributed to trans-rich polymers, and trans vinylene units are more planar and thus less sterically hindered than the cis and react with singlet oxygen faster (Note: addition of a singlet O2 to C=C is a syn addition, requiring both oxygen atoms to have access to C=C). In the case of C8-diCN-PPV, although the trans is expected to be more planar than cis (Figure 9S), it has more steric hindrance than trans of RO-diCN-PPV as CH2 is larger in size than an oxygen atom (Figure 9S). Therefore, both trans and cis in C8-diCN-PPV are highly non-planar and likely have similar reactivity toward singlet O2. 0.4 0 min 0 min 1 min 2 min 4 min 8 min 1 min 0.5 2 min 0.4 0.3 0.2 0.1 Normalized Absorbance Normalized Absorbance 0.6 0 0.3 0.2 0.1 0 10 12 14 16 18 Elution time (min) 20 10 12 14 16 18 Elution time (min) 20 Figure 7S: GPC elugrams for C8-diCN-PPV (left) and RO-diCN-PPV (right) at different total illumination times. In the elugrams of C8-diCN-PPV, the monomer/dimer peaks are not seen as the samples were left on bench for 2 days before being used for GPC. The same change was observed for RO-diCN-PPV samples (not used for the figure shown here). Therefore, the monomer and dimer can evaporate slowly. S6 1.0 0 min 1 0 min 1 min 0.8 0.8 4 min 2 min Normalized intensity 2 min 0.6 0.4 0.2 10 min 0.6 15 min 0.4 0.2 0.0 3.5 3 2.5 Normalized Intensity 1 min 0 2 4.5 4 Chemical shift (ppm) 3.5 Chemical shift (ppm) Figure 8S: The OCH2 regions of 1H NMR spectra of C8-diCN-PPV (left) and RO-diCN-PPV (right) at different total illumination time in minutes. C10H21 O C8H17 NC C7H15 NC C7H15 CH2 H2C CN CN CH2 NC H2C C7H15 C7H15 NC C10H21 H C10H21 O O C10H21 H CN H C7H15 O C10H21 H CH H22C C8H17 CN O O C7H15 O C10H21 O C10H21 C10H21 trans cis trans in C8-diCN-PPV cis in RO-diCN-PPV Figure 9S. Trans and cis Structural units in diCN-PPVs. Groups in circles are too close. Their interactions cause phenyl rings to stay out of the planes of dicyanovinylene. S7 delay time (ms) 0 LN(normalized PL) 1.5 2 2.5 3 3.5 4 -0.2 -0.4 -0.6 y = -0.2379x + 0.2966 -0.8 Figure 10S. Phosphorescence lifetime in nitrogen (4.2 ms). delay time (ms) 0 LN(normalized PL) 1 1.5 2 2.5 3 -0.5 -1 y = -0.4245x + 0.2881 -1.5 Figure 11S. Phosphorescence lifetime in air (2.4 ms). S8 3.5 4 60 PL intensity 50 ROdicnppv 40 C8dicnppv 30 20 10 0 360 410 460 510 560 Wavelength (nm) 610 660 Figure 12S. PL spectra of C8-DiCN-PPV and RO-DiCN-PPV, showing that PL peak intensity of C8-DiCN-PPV is about 50 % as high as that of RO-DiCN-PPV. 350 photoluminescence 300 0 min 250 1 min 20 min 200 40 min 150 60 min 80 min 100 50 0 480 530 580 wavelength (nm) 630 680 Figure 13S. RO-diCN-PPV PL spectra after 0, 1, 20, 40, 60, and 80 minutes of illumination. S9 300 0 min PL intensity 250 1 min 2 min 200 3 min 150 4 min 10 min 100 20 min 30 min 50 0 370 420 470 520 Wavelength (nm) 570 Figure 14S. PL spectra of C8-diCN-PPV after illumination for different amount of time. S10