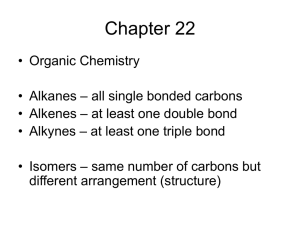
C—C—C—C—C—C—C
advertisement

Honors Chemistry Unit 8A Organic Chemistry Allotropes Isomers Hydrocarbons o Alkanes o Alkenes o Alkynes o Aromatics Alkyl Halides 1 We are learning to: 1. 2. Draw, model and name the hydrocarbon families Recognize isomers of hydrocarbons We are looking for: 1. a. Given a name, draw the structural formula for Alkanes, Cycloalkanes, Alkenes, Cycloalkenes, Alkynes, Cycloalkynes, aromatics. b. Given a structural formula, write the name for Alkanes, Cycloalkanes, Alkenes, Cycloalkenes, Alkynes, Cycloalkynes, aromatics. c. Given a model, write the name for Alkanes, Cycloalkanes, Alkenes, Cycloalkenes, Alkynes, Cycloalkynes, aromatics. 2. Draw and name isomers of given hydrocarbon 2 3 4 5 Parent Chain/Carbon Number Prefixes: Methane CH4 Ethane C2H6 Propane C3H8 Butane C4H10 Pentane C5H12 Hexane C6H14 Heptane C7H16 Octane C8H18 Nonane C9H20 Decane C10H22 Undecane C11H24 Dodecane C12H26 Branches will have one less hydrogen than parent chain formulas given above all prefixes can be made into branches by replacing the –ane ending with–yl. Methyl CH3 Ethyl C2H5 6 Introduction of parent chain and branches: Place a box around the parent chain (longest continuous chain of carbons; it does not have to be in a straight line) 1) C—C—C—C—C | C 3) C—C—C—C—C—C—C | C | C 5) C—C—C | C—C—C—C—C—C—C | C | C 2) 4) 6) C—C—C—C—C—C—C | | C C | C C—C—C—C—C—C—C | | | C C C—C | C C—C—C | C—C—C—C―C | C—C—C—C—C—C—C―C | C—C―C | C 7 Organic Chemistry Hydrocarbons contain Carbon and Hydrogen only If all bonds on the carbons are single bondsdrocarbon Alkanes Have form CnH2n+2 (where n = # of carbons) Ending of the name is “ane” Name Molecular Structural Formula Model Formula CnH2n+2 Methane Ethane Propane ISOMERS!! Butane 2-Methylpropane Basic Rules for Naming: 1) Identify the parent chain and number the carbons so the branches will have the lowest combination of numbers. 2) Name the branches (use –yl ending) in alphabetical order and put the number of its position in front of the branch name. These are written in front of the parent chain name. 3) Put dashes (-) between numbers and letters. 8 Example 1: Draw 3-ethylheptane Example 2: Draw 2,7-dimethylnonane Example 3: Draw 4-ethyl-2,4,5-trimethyloctane Example 4: Draw 3,3,4,4-tetraethyl-2,2,5,5-tetramethylhexane Cycloalkanes All single bonds Have form CnH2n Ends of chain bond together (lose 2 hydrogens) Examples: Draw cyclobutane 9 Draw 1,3-dimethylcyclopentane Draw 1-ethyl-3-methyl-2-propylcyclobutane More practice Naming Alkanes 1) 3) C—C—C—C—C—C—C | C | C 2) C—C—C—C | C | C | C—C—C | C C | C—C—C—C—C—C—C | | | C C C | C 10 Unsaturated Hydrocarbons = not all carbons have 4 single bonds (double or triple bonds) Alkenes Structure contains double bonds Have form CnH2n Name Molecular Structural Formula Model Formula CnH2n Ethene Propene 2-Butene Drawing and naming Alkene structures: (parent chain must include the double bond; number the parent chain to give the double bond the lowest number) 11 Alkynes Structure contains triple bonds Have form CnH2n-2 Name Molecular Formula Structural Formula CnH2n-2 Ethyne 1-Propyne 1-Butyne Naming and drawing Alkynes: 12 Aromatic Hydrocarbons Mu st contain at least one benzene ring Naming Aromatics (when benzene is a branch, the branch is called phenyl ) 13 Name ______________________________________________ Alkane Worksheet Based on the given IUPAC name, give the structural formula for each of the following molecules (number the carbons): Name Structural Formula 1. dodecane 2. 2-methylheptane 3. 2,3,4-trimethylhexane 4. 3-methylpentane 5. 3-ethyl-2,4,4-trimethylnonane 6. 1-butyl,2,4-diethyl-3-methyl cyclobutane 14 Give the IUPAC name for each of the following molecules (number the carbons): Name Structural Formula 1. CH3-CH2-CH2-CH2-CH2-CH2-CH3 2. CH3 CH3-CH-CH2-CH3 3. CH3 CH3 CH3 CH3-CH-CH2-CH-CH-CH3 4. CH2-CH3 CH3 5. 6. CH3 CH3 CH2 CH2 CH3-CH2-CH-CH2-CH2-CH-CH2-CH3 CH3 CH3 CH3 CH2 CH2 CH2 CH3-CH-CH-CH2-CH2-CH-CH2-CH3 15 Introduce Isomers: Using C6H14, how many different ways can you arrange the 6 carbon atoms to form different compounds? Remember, if they are different, they should have different names. 16 Name _____________________________________________ Draw and NAME the 18 isomers of Octane Octane: 1. Methyl heptane: 1. 2. 3. Dimethyl hexane: 1. 2. 3. 4. 5. 6. 17 Ethyl hexane: 1. Trimethyl pentane: 1. 2. 3. 4. Ethyl methyl pentane: 1. 2. Tetramethyl butane: 1. 18 Name ______________________________________________ Alkene Alkyne Assignment Based on the given IUPAC name, give the structural formula for each of the following molecules (number the carbons): Name Structural Formula 1. 3-heptene 2. 3-methyl-butyne 3. 4,5,5-trimethyl-1-hexene 4. 3-ethyl-2,4-dimethyl-2-pentene 5. 5-methyl-1,3-hexadiyne 6. 1,2,4-trimethyl-1-cyclopentene 7. 2,4,5-trimethyl-1,3-cyclohexadiene 19 Give the IUPAC name for each of the following molecules (number the carbons): Name Structural Formula 1. C–C–C=C-C-C 2. C C=C–C–C-C 3. C C C-C-C - C-C=C 4. 5. 6. C C–C-C C C C C C ―C = C – C = C ―C – C C C C C C CΞC-C - C-C -C≡C 7. C C ―C 20 Alkyl Halides: Organic compound with General Formula o o o o – – – – Naming: o Name as if the halogen is a branch Examples: 21 Alkane, Alkene, Alkyne, Cyclos and Aromatic Review Sheet Section 1: Draw the following structures: 1. 1-ethyl-2-methyl-cyclohexane 2. 2,3-dimethyl-1,5-heptadiene 3. 3-bromo-4,5-dichloro-6-iodo-undecane 4. 1-butyl-3-propyl-benzene 5. 3.3.4-trifluoro-1,6-octadiyne 6. 1-ethyl-4-methyl-1,3-cycloctadiene 7. 1-bromo-5,6-dichloro-3-fluoro-2,4-dimethyl-benzene 22 Section 2: Name the following structures C | C—C—C=C | F 1) __________________________________ 2) ___________________________________ 3) ___________________________________ C―C C C C | | C—C—C—C—C—C—C―C | C | C 4) ___________________________________ Br F 5) ___________________________________ 6) ________________________________________ C Cl I | | CΞC—C—CΞC—C―C | I C=C C C―C―C | | C― C ― C―C | | C C | C 7)___________________________________________ C—C—C—C—C—C—C―C―C―C―C | | | | C C C C 23 Information on Doing an “I-Search” Paper to Make-Up a Missed Organic Chem Modeling Lab: 1. An "I-Search” paper is telling the story of what you did in your search, those happenings and facts crucial to your hunt for information on your assigned topic (the topic is the same as the topic for the modeling lab that you missed). You are to write 150 words (one page) for each modeling lab you miss. 2. The paper is to be typed and divided into three parts (each section is to be titled as below): a. What I knew before I started my search. b. The facts I found in my search and what I learned (include uses). c. What I found the most interesting with what I learned. 3. If your make-up paper does not answer these questions, the best score you can receive is half credit. ______________________________________________________________________________________________ Honors Chemistry "Organic Chemistry Modeling Labs" Alkanes – Straight and Branched (2 points, ½ point each) Grade/Initials _______________ 1. hexane _______________ 2. 3-methylpentane _______________ 3. 2, 4-dimethylhexane _______________ 4. 2, 2-dimethylpentane _______________/2 Total points for Straight and Branched Alkanes ______________________________________________________________________________________________ Isomerism (2 points, ½ point each) Grade/Initials: _______________ 1. butane isomer #1 (simple) (0.25 point) name _____________________________________ (0.25 point) _______________ 2. butane isomer #2 (one branch) (0.25 point) name _____________________________________ (0.25 point) _______________ 3. pentane isomer #2 (one branch) (0.25 point) name _____________________________________ (0.25 point) _______________ 4. pentane isomer #3 (two branches) (0.25 point) ______________/2 Total points for Isomerism name _____________________________________ (0.25 point) 24 Unsaturated Hydrocarbons (3 points, ½ point each) Grade/Initials _______________ 1. 1-butene _______________ 2. 2-butyne _______________ 3. 2-methylpropene _______________ 4. 3-methylbutene _______________ 5. 1, 3-pentadiene _______________ 6. 4-methylpentyne _____________ /3 Total points for Unsaturated Hydrocarbons _______________________________________________________________________________________________________ Mixed Aromatic Hydrocarbons, Alkyl Halides and Hydrocarbons (3 points, ½ point each) Grade/Initials _______________ 1. 2-chloropropane _______________ 2. 2-bromo-3,4 -dichloropentene _______________ 3. 5-bromo-1- chloro-4,4-diiodo-5-methyl-2- hexyne _______________ 4. ethylbenzene _______________ 5. 1-ethyl-3-methylbenzene _______________ 6. 1,2,4-trimethylbenzene _____________ /3 Total points for Aromatics and Alkyl Halides _________________________________________________________________________________ Mixed Hydrocarbons _______________ 1. 2, 3, 4-trimethylhexane _______________ 2. propylcyclobutane _______________ 3. Methylbenzene _______________ 4. 1,1-difluoro-2-butyne _______________ 5. 1-iodo-2-methylpropene _______________ 6. 1, 3-pentadiene _______________ 7. 3-bromo-1-chlorobutyne _______________ 8. 1-chloro-2-ethyl-3fluoro-cyclohexene _______________/ 4 Total points for Mixed Hydrocarbon 25 26 Honors Chemistry Unit 8B Organic Chemistry continued Alcohols Ethers Aldehydes Ketones Carboxylic acids Esters Amines 27 We are learning to: 1. Draw, model and name the hydrocarbon families. 2. Recognize isomers of hydrocarbons. 3. Draw, model and name the functional groups We are looking for: 1 & 3 a. Given a name, draw the structural formula for Alkanes, Cycloalkanes, Alkenes, Cycloalkenes, Alkynes, Cycloalkynes, aromatics, alkyl halides, alcohol, ether, aldehydes, ketones, carboxylic acids, esters, and amines. b. Given a structural formula, write the name for Alkanes, Cycloalkanes, Alkenes, Cycloalkenes, Alkynes, Cycloalkynes, aromatics, alkyl halides, alcohol, ether, aldehydes, ketones, carboxylic acids, esters, and amines. c. Given a model, write the name for Alkanes, Cycloalkanes, Alkenes, Cycloalkenes, Alkynes, Cycloalkynes, aromatics, alkyl halides, alcohol, ether, aldehydes, ketones, carboxylic acids, esters, and amines. 2. Draw and name isomers of given hydrocarbon 28 Other Organic Compounds Functional Group – Same Functional Group o o - Alcohols: Organic compound that General Formula Naming: o If no number is present in front of the name – presume the –OH is on carbon number 1. o If 1 -OH group - Examples: o If two or more -OH groups 2= 3= 4= Keep the ane; add the appropriate ending listed above: Examples: 29 Ethers: Organic compounds General Formula: - R and R’ Naming: o– o– o– Examples: 30 Name__________________________________________ Other Organic Compounds – WS I Draw the following compounds (number the parent chain and circle any branches): 1) 1,8 nonanediol 4) dibutyl ether 2) 1, 3, 5 trimethylbenzene 5) 4-decanol 7) 1,2dibromo -1-chloroethane 3) 2-bromo-2-fluoro propane 6) 1-iodocyclobutane 8) 4-ethyl -3,6-difluoro-5-propyl-1-cyclohexene 9) 2,3-dichloro-7,8,9-triiodo-6,7,8-tripropyl-4-dodecene 10) 1,2,3,4,5,6-hexafluoro-1-hexanol 11) Decyl octyl ether 31 32 Other Organic Compounds (Continued) Carbonyl group is a carbon double bonded to an oxygen, C=O Aldehydes: Organic compounds O R–C–H General Form: Naming: Example O C-C-H lanthte Ketones: Organic compounds General Form: O R – C – R’ Naming: Example: O C–C-C 2-propanone 33 Carboxylic Acids: Carboxyl group is a carbon double bonded to an oxygen and single bonded to an oxygen with hydrogen (-OH group) O - C – OH Organic compounds O General Form: R – C - OH Naming o If one carboxyl group - o If more than one carboxyl group For 2 = dioic acid Example: O O OH – C – C – C – C – OH 1,4-butanedioic acid 34 Name__________________________________________ Other Organic Compounds – WS II Draw the following compounds (number the parent chain and circle any branches): 1) 1,5 pentanedioic acid 4) 1,2,4 tributyl benzene 7) 1,2-dichlorocyclohexane 9) 3-iodo-1-propanal 2) 1 heptanal 5) 3,5 dimethyl- 4 propyl octane 3) 3-hexanone 6) Butyl methyl ether 8) Dicyclopentylmethanone 10) 4,5,6,7-tetrabromo -3,8 dichloro -5,6-diethyl-3,8-dimethyl-1,10-decanedioic acid 35 36 More Organic Compounds Continued (again)! Esters: Organic compounds O R - C - OH (Acid) O R - C - O - R’ (Ester) Naming: Parent Chain o o o o Branches o o Example: O C - C - O - C - C-C 2 1 1 2 3 (Parent) (Alkyl branch) Parent Chain: Branch: Final Name:_______________________________________ 37 Example: O C - C - C - C - O - C - C 4 3 2 1 1 2 Parent Name: Branch: Final Name: __________________________________ Draw: Pentyl Hexanoate 38 Amines: Organic compounds based on NH3 R - N - R” R’ Naming: Primary amine: (______ hydrogen is replaced by an alkyl group) C–N–H H Name: ___________________________ Secondary amine: (____ hydrogens are replaced by alkyl groups) C–N–C - C H Name: _____ ______ _______________ Tertiary amine: (____ hydrogens are replaced by alkyl groups) C–N–C - C C–C-C 39 Name: __________________________________________________ Ester and Amine Worksheet Draw each of the following compounds: 1) ethyl butanoate 2) propyl-benzoate 3) propylamine 4) diethylamine 5) butylethylpropylamine 6) hexyloctylamine 7) methyl-2-methylhexanoate 8) propyl pentanoate 9) phenyl butanoate 40 41 Organic Compounds Family Functional Group Ending Naming Alkane Alkene Alkyne Aromatic Alkyl halides Alcohol 42 Family Functional Group Ending Naming Ether Aldehyde Ketone Carboxylic Acid Ester Amine 43 Name: __________________________________________________ Mixed Worksheet Draw each of the following structures: 1) 1-chloro-2-pentyl-4-propyl benzene 3) 2-iodo-3-methyl butanedial 5) propyl undecyl ether 7) 2,5-dimethyl-1-cyclopentanone 9) 1,4,9,11-dodecanetetrol 2) 5-fluoro-2-octyne 4) 5,5-dibromo-1,1,1-trifluoro-8-ethyldecane 6) 1,3,6-heptatriene 8) 2,3,4,5,6-pentafluoro-1-nonanal 10) 4-bromo-5-butyl-3-ethyl-1-cycloheptyne 44 45 Name ___________________________________________________________ Review for Organic Test Part 1: Draw the following structures: 1. 1,4,7-octanetriol 2. 2,4-dibromo-3-fluoro-1-hexene 3. heptyl hexyl ether 4. butyl pentanoate 5. 3-chloro-5-ethyl heptanal 6. dodecane 7. 4-bromo-5,5-difluoro-3-decanone 8. butyl ethyl pentyl amine 9. 2,3-dimethyl 4-nonene 10. 1-bromo-4-butyl-2-ethyl-3 propyl benzene 11. 1,4,5,trichloro-2-pentyne 12. 2,3,5,7,9 pentabromo-1,4,8-trichloro-6,6 diiodo undecane 46 Part 2: Name the following structures 47 48 Part 3:Model Review Draw and name each of the models: #1 #2 Name Name #3 #4 Name Name #5 #6 Name Name 49 #7 Name #8 Name #9 #10 Name Name #11 #12 Name Name 50 Honors Chemistry Lab Practical "Organic Chemistry" Alcohols and Ethers (3 points, ½ point each) Grade/Initials _______________ 1. 2-propanol (rubbing alcohol) _______________ 2. Butyl ethyl ether _______________ 3. 3-ethyl-4-methylpentanol ______________ 4. Dicyclopentyl ether _______________ 5. Iodo phenyl ether _______________ 6. 1-fluoro-2-methyl-1,1,2,3-propanetetrol ____________/3___ Total points for Alcohols and Ethers _____________________________________________________________________________________________________ Aldehydes and Ketones Grade/Initials (3 points; ½ each) _______________ 1. propanone (acetone) _______________ 2. 2-methylpropanal _______________ 3. 2,2-dimethylpentanal _______________ 4. methanal (formaldehyde) _______________ 5. 2-pentanone _______________ 6. 5-chloro-6,6-dimethyl-3,4-heptadione _________/3______ Total points for Aldehydes and Ketones ____________________________________________________________________________________________________ Grade/Initials (3 points; ½ each) Carboxylic Acids _______________ 1. butanoic acid _______________ 2. 3-methylpentanoic acid _______________ 3. 3, 3-difloro-4-ethylhexanoic acid _______________ 4. 2-butylpropanedioic acid _______________ 5. 4-ethyl-2-methylheptanoic acid _______________ 6. benzoic acid (hint, you need to add a carbon off of the ring for the carboxyl group) _________/3____ Total points for Carboxylic Acids \ 51 Grade/Initials (3 points; ½ each) Esters and Amines _______________ 1. butylamine _______________ 2. ethylpropylamine _______________ 3. dimethyl pentylamine _______________ 4. ethylmethanoate Design one of the following for 2 points, if right it is placed on the board and other teams may not use it: _______________ 5. design an ester name _____________________________________ with 5 carbons total (can not be straight chain)(Instructor checks) _______________ 6. design an ester name _____________________________________ with 7 carbons total (can not be straight chain)(Instructor checks) ________/3_______ Total points for Esters/Amines _____________________________________________________________________________________________________ ALL Mixed Up Grade/Initials _______________ 1. 4-methyl-2,3-octanediol _______________ 2. 3-chloro-3-ethyl-2,2,4-trimethylpentane _______________ 3. 3-propylhexanoic acid ______________ 4. Butyl methyl propyl amine _______________ 5. Pentyl propyl ether _______________ 6. 1-bromo-4-iodo-2-propylbenzene _______________ 7. 6,6-difluoro-3-methyl-3-propyl-1,4-hexadiyn _______________ 8. 3-methyl-2,2-difluorobutanal _______________ 9. 4-ethyl-6-iodo-3,3-dimethyl-1-cyclohexene _______________ 10. Propylbenzoate _______________ 11. 5-fluoro-2-methyl-3-hexanone _______________ 12. 1-fluoro-2-methyl-3-hexanone Total Points = /6 52