Corrosion lab – potfolio task 1
advertisement

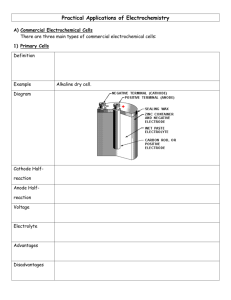
Corrosion lab The most popular type of corrosion is aqueous corrosion. This is an electrochemical process where four important points are essential: An anode A cathode An electrolyte conductor An electronic conductor Before the corrosion lab, Agar-agar gel containing 3% sodium chloride was prepared. Then, Potassium ferricyanide solution and phenol phthalein solution was added and the final solution was plates into Petri dishes. These Petri dishes was used in tests 2 to 7. Test 1: In this test a metallic triangle was used. After the process, it is possible to identify, as shown by Figure 1 below, a blue area. This area indicates the anode, where corrosion is running. In the anode the iron oxidation occurs and produce Fe2+. A pink circle could be seen too, which is the cathode. In the cathode the oxygen reduction occurs and produce OH-. This corrosion method occurs due to the fact that the offer of oxygen is not the same in all regions and next to the cathode is the area with less oxygen. In addition to this, between the pink circle - cathode and the blue area – anode is where corrosion product is forming. Figure 1 – Metallic triangle corroded during test 1 Test 2: In this test a Petri dish was used and one curved iron nail was placed into the agar-agar solution, as shown by Figure 2 below. After the process, it is possible to identify an blue area. This area indicates the anode, where iron oxidation occurs and produce Fe2+. The anode is localized there due to the fact that in where the nail suffer deformation and, then, it is favorable to the oxidation due to thermodynamic factors. Two pink areas could be seen too, which are the cathode. In the cathode the oxygen reduction occurs and produce OH-. This illustrates a stress corrosion. Figure 2 – System used for test 2: Petri dish and iron nail which suffered corrosion Test 3 In this test a Petri dish was used and one iron nail was placed into the agar-agar solution, as shown by Figure 3 below. After the process, it is possible to identify an blue area. This area indicates the anode, where iron oxidation occurs and produce Fe2+. The anode is localized in the middle of the nail where is easier to suffer corrosion. This probably occurred because the ends of the nail where cleaner than the middle and due to thermodynamic. Two pink areas could be seen too, which are the cathode. In the cathode the oxygen reduction occurs and produce OH-. Another important aspect to remember that this corrosion, like in test 1, is spontaneous. This is true due to the fact that the electronegativity iron is equal to -0.473V. As this constant is negative, the reaction occurs without any intervention. Figure 3 – System used for test 3: Petri dish and iron nail which suffered corrosion Test 4 In this test a Petri dish was used and one iron nail half zinc plated at the pointed end was placed into the agar-agar solution, as shown by Figure 4 below. After the process, it is possible to identify a pink area, which is the cathode. In the cathode the iron reduction occurs and produce Fe. One white area could be seen too, the anode, where zinc oxidation occurs and produce Zn2+. During this process the zinc was corroded and not the iron, due to the fact that the Zn is more electronegative than iron, and then, Zn is more reactive. The electronegativity of the zinc is approximately -1.0V and for the iron this constant is equal to -0.473V. So, if the zinc suffer oxidation and the steel reduction, the sum of the electronegativity of the half-reactions is negative (-1.0V + 0.473V = - 0.527V) and this process is spontaneous. In this case that zinc protect iron from corrosion, the first metal is named sacrificial anode and this technique is used to avoid corrosion of metal using a cheaper one to protect it. This is an example of galvanic corrosion. Figure 4 – System used for test 4: Petri dish and iron nail half zinc plated at the pointed end which suffered corrosion Test 5 In this test a Petri dish was used and one iron nail half copper plated at the pointed end was placed into the agar-agar solution, as shown by Figure 5 below. After the process, it is possible to identify two pink areas, which are the cathode. In the cathode the copper reduction occurs and produce Cu. One blue area could be seen too, the anode, where iron oxidation occurs and produce Fe2+. During this process the iron was corroded due to the fact that the Fe is more electronegative than Cu, and then, Fe is more reactive. The electronegativity of the iron is approximately -0.473V and for the copper this constant is equal to -0.246V. So, if the iron suffer oxidation and the copper reduction, the sum of the electronegativity of the half-reactions is negative (-0.473 + 0.246V = - 0.227V) and this process is spontaneous. In conclusion, copper do not protect the substrate and if the surface is damage the effects could be worse than the effects that occur without copper. Figure 5 – System used for test 5: Petri dish and iron nail half copper plated at the pointed end which suffered corrosion Test 6 In this test a Petri dish was used and one iron nail and one zinc strip were placed into the agar-agar solution, as shown by Figure 6 below. After the process, it is possible to identify a pink area around the nail, which is the cathode. In the cathode the iron reduction occurs and produce Fe. One white area could be seen too around the zinc strip, the anode, where zinc oxidation occurs and produce Zn2+. During this process the zinc was corroded and not the iron, due to the fact that the Zn is more electronegative than iron, and then, Zn is more reactive. The electronegativity of the zinc is approximately -1.0V and for the iron this constant is equal to -0.473V. So, if the zinc suffer oxidation and the steel reduction, the sum of the electronegativity of the half-reactions is negative (-1.0V + 0.473V = 0.527V) and this process is spontaneous. In this case that zinc is a sacrificial anode and electrons ate transferred from iron nail to zinc strip by wires and clamps connected in the system. Figure 6 – System used for test 6: Petri dish, iron nail and zinc strip Test 7 In this test a Petri dish was used and one iron nail half zinc plated at the pointed end and one zinc strip were placed into the agar-agar solution, as shown by Figure 7 below. After the process, it is possible to identify a pink area around the nail, which is the cathode. In the cathode, iron and copper reduction occur and produce Fe and Cu. One white area could be seen around the zinc strip, the anode, where zinc oxidation occurs and produce Zn2+. During this process the zinc was corroded and not the iron or the copper, due to the fact that the Zn is more electronegative than iron and more electronegative than copper too. Then, Zn is more reactive. The electronegativity of the zinc is approximately -1.0V, for the iron this constant is equal to -0.473V and for the copper this constant is equal to 0.246V. So, if the zinc suffer oxidation and the steel reduction, the sum of the electronegativity of the half-reactions is negative (Iron and Zinc: -1.0V + 0.473V = 0.527V and Copper and zinc: -1.0V + 0.246V = - 0.754V) and this process is spontaneous. In this case that zinc (sacrificial anode) protect iron and copper from corrosion. Figure 7 – System used for test 7: Petri dish, iron nail half copper plated at the pointed end and zinc strip