Aldehydes and Ketones
advertisement

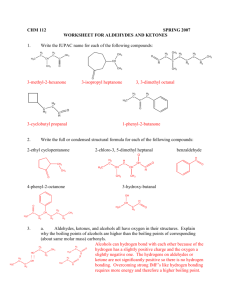
King Saud University Chemistry Department ALDEHYDES AND KETONES 145 Chem 1 King Saud University Chemistry Department ALDEHYDES: STRUCTURE AND NOMENCLATURE • General formula: RCHO or RCH=O • The aldehyde group is always at the end of a chain • IUPAC system: Select the longest continuous carbon chain that contains the C=O group and replace the ending by the suffix al. • The CHO group is assigned the number 1 position and takes precedence over other functional groups that may the present such as –OH, C=C for example. 145 Chem 2 King Saud University Chemistry Department O O O O H H3C H H H H3CH2CH2C H3CH2C Common Formaldehyde Acetaldehyde Propionaldehyde Butyraldehyde IUPAC Methanal Ethanal Propanal O H3C CH H Cl IUPAC 2-ChloroPropanal 145 Chem Butanal O H2 C C HO C H2 H2 3-HydroxyPropanal H O H H C C H3C H H 2-Butenal 3 King Saud University Chemistry Department • Aromatic aldehydes are usually designated as derivatives of the simplest aromatic aldehyde, Benzaldehyde. O O H H O 2N p-Nitrobenzaldehyde O Benzaldehyde OH O H o-Hydroxybenzaldehyde Salicylaldehyde 145 Chem H H3CO P-Methoxybenzaldehyde Anisaldehyde 4 King Saud University Chemistry Department KETONES: STRUCTURE AND NOMENCLATURE • General formula: RCOR’ (R and R’=alkyl or aryl) • Common name: listing the alkyl substitutents attached to the carbonyl group, followed by the word ketone. • IUPAC system: relpace the ending –e by the suffix –one. The chain is numbred in such a way as give the lowest number to the C=O group. 145 Chem 5 King Saud University Chemistry Department O H3C Common C O CH3 H3C O C C6 H 5 H3C C CH=CH 2 H5C6 C C6H5 Dimethyl Ketone Methyl phenyl ketone Methyl vinyl Diphenyl ketone Acetone Acetophenone ketone Benzophenone Propanone Phenyl ethanone 3-Buten-2-one Diphenylmethanone IUPAC O O C2 H 5 O O OH CHO C C2 H 5 1-Cyclopentylpropanone 3-Ethyl-2-hydroxycyclohexanone 145 Chem 5-Oxohexanal 6 King Saud University Chemistry Department PHYSICAL PROPERTIES OF KETONES ANDALDEHYDE O C + O C O - C C O • Because the polarity of the carbonyl group, aldehydes and ketones are polar compounds. • Dipole-dipole attractions, although important, are not as strong as intractions due to hydrogen bonding. As a result, the boiling points of aldehydes and ketones are higher than those of nonpolar alkanes, but lower than those of alcohols. • The lower aldehydes and ketones are soluble in water. C 145 Chem O H O H O C 7 King Saud University Chemistry Department PREPARATION OF ALDEHYDES AND KETONES 1- Oxidation of alcohols RCH2OH O CrO3/pyridine or Cu/ heat R H O R2CHOH CrO3/pyridine or Cu/ heat R R 2- Ozonolysis of alkenes A A 145 Chem A A 1] O3 2]Zn/ H2O A O A + A O A 8 King Saud University Chemistry Department PREPARATION OF ALDEHYDES AND KETONES 3- Hydration of alkynes C C + HO H H2SO4,HgSO4 H H C C OH an enol unstable 4- Friedel Grafts acylation H O carbonyl more stable O O + 145 Chem AlCl3 Cl 9 King Saud University Chemistry Department REACTIONS OF ALDEHYDES AND KETONES Nucleophilic addition Reactions 1- Reduction of carbonyl group 2H2/Pd H3C OH H3C OH H H3C O 1] NaBH4 2]H2O 145 Chem 10 King Saud University Chemistry Department 2- Addition of Grignard Reagents: Formation of alcohols O + R H R'MgX O H3C 2] H3O+ R + C2H5MgX H 2] H3O+ H3C CH R' R''MgX 2] H3O+ R' C2H5 OH 1] dry ether + CH OH 1] dry ether O R OH 1] dry ether R C R'' R' CH3 O 1] dry ether + 145 Chem CH3MgX 2] H3O + OH 11 King Saud University Chemistry Department 3- Oxidation reaction O O or R Ar H H O O KMnO4 or or K2Cr2O7 R OH Ar OH 4- Addition of Hydrogen Cyanide: Formation of cynohydrins O R OH + HCN R' R R' cyanohydrin CN O CN H O OH + 145 Chem OH Benzaldehyde cyanohydrin + HCN HCN CN 12 King Saud University Chemistry Department 6- Addition of alcohols: O C2H5OH,H+ H3C OH H3C H Ph CH3 145 Chem C2H5OH,H H3C OH Ph OC2H5 H Hemiacetal C2H5OH,H+ Ketone OC2H5 H Aldehyde O OC2H5 + Acetal OC2H5 + OC2H5 C2H5OH,H Ph OC2H5 CH3 CH3 Hemi ketal Ketal 13 King Saud University Chemistry Department 7- Addition of Ammonia and Ammonia Derivatives N NH2 NH2NH2 -H2O H3C H hydrazone N NHPh PhNHNH2 O H3C H3 C H phenyl hydrazone H H N N OH Aldehyde NH2OH H Oxime H imine H3C NH NH3 H3C 145 Chem 14 King Saud University Chemistry Department Question 1- The correct name of the following compound is: A) 3-hydroxyhexanal B) 3-hydroxy-4-hexenal C) 4-hydroxy-5-hexenal D)3-hydroxy-1-hexenal 2- The structure of Acetal is: O A) C2H5 B) OH C) C2H5 145 Chem O OH O C2H5 OH O C H H 2 5 O D) C2H5 C2H5 O 15 King Saud University Chemistry Department 3- Reaction of phenyhydrazine with carbonyl compounds (aldyhydes or ketones) gives: A) Oxime B) Phenylhydrazone C) ImineD) Hemiacetal 4 - Which of the following compounds has the highest boiling point? 145 Chem 16 King Saud University Chemistry Department Thank you for your attention 145 Chem 17