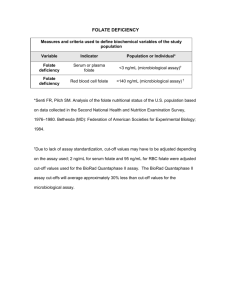
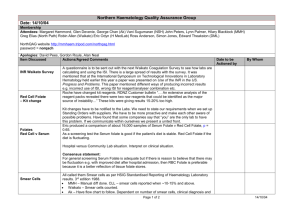
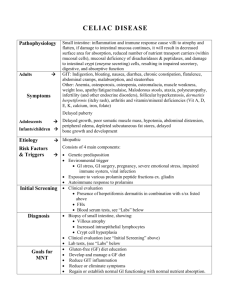
Word Version Folate Testing Review Report
advertisement

MBS Reviews FOLATE TESTING REPORT February 2014 February 2014 TABLE OF CONTENTS Section Page TABLE OF CONTENTS .................................................................................................................... 2 LIST OF ABBREVIATIONS ............................................................................................................ 4 EXECUTIVE SUMMARY ................................................................................................................ 5 PURPOSE OF THE REVIEW ......................................................................................................................................... 5 FOLATE TESTING ...................................................................................................................................................... 5 CONCERNS ABOUT FOLATE TESTING......................................................................................................................... 5 REVIEW METHODOLOGY .......................................................................................................................................... 5 STAKEHOLDER CONSULTATION ................................................................................................................................ 6 SUMMARY OF FINDINGS ........................................................................................................................................... 6 APPROPRIATE CLINICAL INDICATIONS FOR FOLATE TESTING .................................................................................... 7 CONCLUSIONS .......................................................................................................................................................... 8 1 BACKGROUND ON FOLATE TESTING................................................................................. 9 1.1 DESCRIPTION OF CURRENT SERVICES .......................................................................................................... 9 1.2 THE CLINICAL FLOWCHARTS ..................................................................................................................... 13 2 REVIEW METHODOLOGY ..................................................................................................... 15 2.1 SECONDARY DATA ANALYSIS .................................................................................................................... 15 2.2 GUIDELINE CONCORDANCE ....................................................................................................................... 15 2.3 SYSTEMATIC LITERATURE REVIEW FOR CLINICAL EVIDENCE..................................................................... 16 2.4 SYSTEMATIC LITERATURE REVIEW FOR ECONOMIC EVIDENCE .................................................................. 18 3 SECONDARY DATA ANALYSIS ............................................................................................. 20 3.1 MBS ITEM NUMBER USAGE AND EXPENDITURE ......................................................................................... 20 3.2 AGE AND GENDER PROFILE OF PATIENTS ................................................................................................... 23 3.3 FREQUENCY OF TESTING BY PATIENT ........................................................................................................ 24 3.4 PROFILE OF PROVIDERS REQUESTING FOLATE/VITAMIN B12 TESTING SERVICES ....................................... 25 3.5 FREQUENCY OF REQUESTS FOR TESTING BY PROVIDER .............................................................................. 26 4 REVIEW OF GUIDELINES RELEVANT TO FOLATE TESTING .................................. 28 4.1 AUSTRALIAN GUIDELINES ......................................................................................................................... 28 4.2 INTERNATIONAL GUIDELINES .................................................................................................................... 28 4.3 OTHER REPORTS ........................................................................................................................................ 29 5 REVIEW OF THE CLINICAL EVIDENCE FOR FOLATE TESTING ............................ 31 5.1 EVIDENCE BASE ........................................................................................................................................ 31 MBS Reviews – Folate Review Report Page 2 February 2014 5.2 APPROPRIATE CLINICAL INDICATIONS FOR FOLATE TESTING ..................................................................... 31 5.3 EVIDENCE THAT TESTING FOLATE LEVELS IMPROVES HEALTH OUTCOMES ................................................ 31 5.4 RISKS/HARMS ASSOCIATED WITH FOLATE TESTING ................................................................................... 32 5.5 QUALITY OF TESTING ................................................................................................................................ 32 6 REVIEW OF THE ECONOMIC EVIDENCE FOR FOLATE TESTING ......................... 33 6.1 COSTING STUDIES OR ECONOMIC ANALYSES RELEVANT TO FOLATE TESTING ............................................ 33 7 FINDINGS AND CONCLUSIONS ............................................................................................ 34 7.1 CURRENT USAGE OF FOLATE AND/OR VITAMIN B12 TESTING SERVICES IN AUSTRALIA ............................. 34 7.2 CLINICAL GUIDANCE ON FOLATE TESTING................................................................................................. 35 7.3 RELATIONSHIP BETWEEN TESTING FOR FOLATE LEVELS AND HEALTH OUTCOMES .................................... 35 7.4 HARMS ASSOCIATED WITH FOLATE TESTING ............................................................................................. 36 7.5 QUALITY OF FOLATE TESTING ................................................................................................................... 36 7.6 COST IMPLICATIONS OF FOLATE TESTING .................................................................................................. 36 7.7 CONCLUSIONS ........................................................................................................................................... 36 APPENDIX 1 – REFERENCES............................................................................................................ 38 APPENDIX 2 – REVIEW CONSULTATION COMMITTEE MEMBERS ............................................... 42 APPENDIX 3 – MBS INFORMATION................................................................................................ 43 APPENDIX 4 – SEARCH TERM STRATEGY ...................................................................................... 44 APPENDIX 5 – TOOLS FOR ASSESSING THE EVIDENCE IN THE SYSTEMATIC REVIEW ................ 49 MBS Reviews – Folate Review Report Page 3 February 2014 LIST OF ABBREVIATIONS AIHW Australian Institute of Health and Welfare ANZFSC Australian and New Zealand Food Standards Code CMFM Comprehensive Management Framework for the MBS CVD Cardiovascular disease FDA Food and Drug Administration HTA Health technology assessment MBS Medicare Benefits Schedule MMA Methylmalonic acid MSAC Medical Services Advisory Committee NHMRC National Health and Medical Research Council NTD Neural tube defects PASC Protocol Advisory Sub-Committee (of MSAC) PBS Pharmaceutical Benefits Scheme PICO Population, Intervention, Comparator, Outcomes RBC Red blood cells RCC Review Consultation Committee RCT Randomised controlled trials RDA Recommended dietary allowance MBS Reviews – Folate Review Report Page 4 February 2014 EXECUTIVE SUMMARY In the 2011-12 Budget, the Australian Government announced a further commitment to the Comprehensive Management Framework for the Medicare Benefits Schedule [MBS] (CMFM), to continue the systematic review of MBS items to ensure that they reflect contemporary evidence, improve health outcomes for patients and represent value for money. MBS Reviews aim to ensure the clinical and financial sustainability of the MBS. Reviews assess specific MBS services (i.e. MBS items) and associated policy issues in a focussed, fitfor-purpose, evidence-based process. Findings recognise that the MBS funding should align with contemporary evidence, reflecting appropriate patient groups and best clinical practice. The Reviews have a primary focus on improving health outcomes and the financial sustainability of the MBS, through the following criteria: assess patient safety risk; identify services that have limited health benefit and/or are used inappropriately; be evidence-based and fit-for-purpose; be conducted in consultation with key stakeholders including, but not limited to, the medical profession and consumers; include opportunities for public submission; and use Government resources efficiently. Purpose of the review This Review Report outlines the rationale behind conducting the review of the MBS items relevant to folate testing services (refer to Appendix 3 for MBS item descriptor) and the process undertaken to identify and appraise the available information on the MBS item to ensure that it reflects contemporary evidence, improves health outcomes for patients, and represents value for money. Folate testing Folate is a water soluble B vitamin (also known as vitamin B9) that occurs naturally in food. Folate deficiency can be the result of nutritional deficiency, increased requirements, impaired absorption, or other gastrointestinal causes. Deficiency is associated with a wide spectrum of haematologic, neurologic and psychiatric disorders. The diagnosis of folate deficiency has traditionally been based on measuring serum or red blood cell folate. Concerns about folate testing Concerns raised with folate testing relate to the increase in the number of claims and benefits paid for MBS item numbers 66599 and 66602, both of which relate to testing vitamin B12 or folate levels. Given the widespread testing of folate/vitamin B12 by general practitioners, this review is focused on identifying appropriate clinical indications for medically necessary folate testing, and determining whether testing should be limited to certain high risk groups. Review methodology The review methodology comprised of consulting with key stakeholders; developing a review protocol, which outlined the detailed review methodology (including specifying the key MBS Reviews – Folate Review Report Page 5 February 2014 clinical/research questions for the systematic review, preparing the clinical flowcharts, and documenting the economic analysis strategy); analysing secondary data sources (Medicare Australia); conducting an evidence-based systematic literature review on folate; and undertaking an assessment and analysis of the evidence to draw conclusions in relation to the clinical/research questions. Stakeholder consultation Stakeholder engagement is a pivotal part of the MBS Reviews process, particularly as feedback helps inform Review Reports. During the review process, stakeholders were informed of the progress of the MBS items being considered. This included ensuring that relevant documents were released for public consultation at the appropriate time and that comments were incorporated into the review process. As part of the MBS Review process, the Department established a Review Consultation Committee (RCC). The RCC is a time-limited committee of nominated representatives, established to provide advice to the Department. A list of RCC members is found at Appendix 2. Summary of findings Current usage of folate and/or vitamin B12 testing services in Australia Over the past 10 years, the number of claims for MBS item 66599 has more than doubled (+119%) from 282,531 in 2003/04 to 618,744 in 2012/13. Over the same timeframe the number of claims for MBS item 66602 has had an even greater increase (+307%) from 522,980 to 2,129,051. The increase in benefits paid for both items reflects the increase in claims (+120% for item 66599 and +309% for item 66602). While total benefits increased significantly, the proportion of benefits paid to each state and territory remained relatively constant over the ten-year period. The highest proportion of benefits paid over the past ten years was in New South Wales (34% and 38% of total benefits for 66599 and 66602, respectively), followed by Victoria (30% for 66599 and 28% for 66602). An analysis was conducted of the number of services per capita (i.e. per 100,000 population). Across Australia, there were 2,666 claims for item 66599 per 100,000 people enrolled in Medicare in 2012/13 and 9,172 claims per 100,000 for item 66602. South Australia had the highest per capita rate of claiming for item 66599, while the Northern Territory had the lowest. For item 66602, the highest number of claims per capita was for NSW and Victoria, while Tasmania had the lowest. MBS item numbers 66599 and 66602 are claimed by both males and females; however, females had a higher number of tests at all ages, except in the youngest age category (< 5 years). Females also had a steeper increase in testing volume than males, with the largest difference between genders in the 15-24 and 25-34 year age groups. For item 66599, the number of tests being performed in people aged 45 years and over was 76% for males and 64% for females. For item 66602, 71% of claims for males were aged 45 years and over versus 60% for females. For both MBS items, there was an increase in the overall number of patients tested between 2008/09 and 2012/13. However, there was very little change in the proportion of patients receiving either one test per year (approximately 91%), two tests per year (approximately 8%), or three or more tests per year (approximately 1%). These data suggest that the majority MBS Reviews – Folate Review Report Page 6 February 2014 of folate/vitamin B12 testing services are being undertaken for the purposes of screening/testing rather than monitoring. Over the five-year time period from 2008/09 to 2012/13, there were no material changes in the pattern of requesting providers. General practitioners and other medical practitioners accounted for approximately 71% and 67% of all providers requesting item 66599 and item 66602, respectively. Approximately 14% of providers requesting folate/vitamin B12 testing were internal medicine consultant physicians. There was a large variety of other providers requesting services, but they each accounted for less than 4% of provider counts. For both items, there was an increase over the period 2008/09 to 2012/13 in the overall number of providers requesting folate/vitamin B12 testing. The majority of providers (97% and 88% for item 66599 and 66602, respectively) requested 100 or fewer tests per year. There were a small number of providers that requested more than 400 tests per year (601 providers in 2012/13). Appropriate clinical indications for folate testing Although there are a large number of clinical practice guidelines that recommend folate supplementation, particularly in pregnant women, they do not specifically mention folate testing, which implies that testing may not be necessary in these populations. Guidelines that mention folate testing apply to particular populations. For example, NICE guidance on dementia recommends that measurement of serum folate levels are included in a basic dementia screen, performed at the time of presentation. Guidance on the diagnosis and management of chronic fatigue syndrome/myalgic encephalomyelitis from the Royal College of General Practitioners advises that folate levels should not be carried out unless a full blood count and mean cell volume show a macrocytosis. A July 2013 report from Health Quality Canada used expert consultation to identify health conditions where folate deficiency may be of concern and where folate testing may be appropriate. Based on the expert consultation, the Ontario Health Technology Advisory Committee (OHTAC) recommended that red blood cell folate testing be restricted to individuals with: low haemoglobin levels and a high mean corpuscular volume; suspected gastrointestinal disorders causing malabsorption or suspected malnutrition of any cause. Evidence that testing folate levels improves health outcomes The main function of folate is to help form RBCs and produce DNA. Folate also prevents NTD during fetal development. A deficiency in folic acid can lead to increased homocysteine levels in plasma since folate is necessary for the conversion of homocysteine to methionine. In addition, deficiency can result in perturbation of the metabolic pathway of conversion of homocysteine to methionine with consequent disruption of DNA synthesis caused by thymidine lack resulting in megaloblastic anaemia, as well as other adverse effects on the nervous system and other organs. However, folate deficiency is much less common since the introduction of mandatory fortification with folate of wheat flour in Australia in September 2009. A retrospective study to determine the impact of the fortification program on blood folate levels reported that the prevalence of low serum folate levels has decreased from 9.3% in 2007 to 2.1% in 2010 and the prevalence of low RBC folate levels decreased from 3.4% to MBS Reviews – Folate Review Report Page 7 February 2014 0.5%. Due to the low prevalence of folate deficiency, the clinical utility of folate testing is questionable. No definitive conclusions can be drawn about the effectiveness of folate testing since no prospective trials have been conducted to directly assess the impact of testing on health outcomes in healthy populations or in patients with chronic disease associated with folate deficiency. Although retrospective studies do not meet the inclusion criteria for this review, several retrospective studies (low level evidence) were identified that examined the clinical utility of folate testing in the United States. Given the low rates of serum folate deficiency and the lack of change in management based on deficient results, the authors of the studies concluded that in folic acid fortified countries, serum folate testing has low utility for inpatients and emergency department patients or routine testing in clinical practice for patients with anaemia or dementia. No trials designed to directly measure the risks or harms associated with folate testing were identified. However, folate testing relies on a blood draw, which is a safe procedure. It is likely that the consequences of inaccurate or inappropriately interpreted folate test results, such as a false positive, are relatively small. Folate supplements are generally considered safe when taken in amounts that are not higher than the recommended dietary allowance. A poor quality systematic review sought to compare the effectiveness of serum versus red cell folate. The review found that serum folate appeared to be a superior marker of folate status in vitamin B12 deficiency and may have more sensitivity in responding to changes in folate intake (supplements or fortification) than red cell folate. Both tests appeared to predict NTD risk equally. Serum folate also has the advantage of being influenced by fewer analytical variables. In an evaluation of serum and red cell folate versus homocysteine, there was no evidence for the better performance of red cell folate and, of the two, serum folate appeared superior. Studies also demonstrated that very few patients would have their clinical outcome altered by the measurement of red cell folate in addition to serum folate. The authors concluded that overall, as a routine test of folate status, serum folate appears to offer the best combination of test cost and clinical information. There was very limited and poor quality evidence relating to the cost of folate testing. In one retrospective study from the United States, the authors concluded that the exceptionally low utility of serum folate testing makes the costs associated with these tests excessive. Conclusions There has been a substantial increase in the number of claims for folate/vitamin B12 testing over the past ten years. Analysis of MBS data indicates that the majority of vitamin B12 testing services are requested by GPs and OMPs for the purposes of screening or testing, rather than follow-up monitoring. There are no Australian clinical practice guidelines that either advocate or recommend against routine testing for folate in any patient population. However, there is guidance from the UK recommending measurement of serum folate levels in a basic dementia screen. There is also guidance from the UK that folate levels should not be carried out in patients with chronic fatigue syndrome/myalgic encephalomyelitis unless a full blood count and mean cell volume show a macrocytosis. There are no recommendations on the frequency of folate testing and there is no prospective evidence regarding the clinical utility of folate testing in any population. However, low level evidence from retrospective studies suggests that serum folate testing has low utility in folic acid fortified countries. MBS Reviews – Folate Review Report Page 8 February 2014 1 BACKGROUND ON FOLATE TESTING 1.1 Description of current services This section describes folate and folate testing, recommended folate status, and the population groups and clinical conditions/risk factors in which folate testing is recommended. 1.1.1 Description and function of folate Folate is a water soluble B vitamin (also known as vitamin B9) that occurs naturally in food. Folate also refers to folic acid otherwise known as pteroyl glutamic acid (PGA). In the body, folate is typically in the reduced form tetrahydrofolate (THF). Food folates are hydrolysed to monglutamate forms in the gut to allow absorption in the intestine.(1) Absorbed folate is transported to the liver, which contains about half the bodies stores of folate(2), while the rest is transported via the systemic circulation to body tissues. 1.1.2 The functions of folate in the human body Folate is a substrate (and vitamin B12 a co-enzyme) for the conversion of homocysteine (a homologue of the amino acids cysteine and methionine) to methionine (an amino acid and one of the 20 building blocks of proteins) by the enzyme methionine synthase (Figure 1.1).(3, 4) More importantly, this pathway is closely linked to the generation of thymidine which is vital for deoxyribonucleic acid (DNA, i.e. the building block of the human body which carries genetic information) synthesis. Figure 1.1: The metabolic reactions that require vitamin B12 and folate (folic acid)(5) 1.1.3 Folate requirements Folate requirements can vary according to lifestyle behaviours (e.g. smoking and alcohol intake), genetic variations (e.g. C667T polymorphism of methylenetetrahydrofolate), use of certain medications (e.g. antiepileptic drugs) and pregnancy. The bioavailability of folate in foods ranges from 50-60%, whilst that of folic acid used to fortify foods (or as a supplement) is about 85%.(6, 7) In adults in Australia the recommended daily intake for folate is 400 µg/day. For women during pregnancy this increases to 600 µg/day and during lactation to 500 µg/day.(8) 1.1.4 Causes of folate deficiencies Folate deficiency may occur due to inadequate nutritional intake, increased requirements (e.g. in pregnancy) or impaired absorption (see Table 1.1). In general, folate deficiency is most MBS Reviews – Folate Review Report Page 9 February 2014 often the result of decreased intake and is more common in developing and socioeconomically distressed countries. Situations in which inadequate intake is further compounded occur when there is an increased folate requirement arising due to pregnancy, lactation, and prematurity. Other conditions associated with increased cell turnover, such as leukaemia’s, aggressive lymphomas and other tumours associated with a high proliferative rate, can also cause increased folate demand.(9) Table 1.1: Causes of folate deficiency Nutritional deficiency Alcoholism(10, 11) Malnutrition(12) and avoidance of fortified bread due to coeliac disease(13, 14) Increased requirements Due to pregnancy and lactation(15-18) Impaired absorption or metabolism Gastrectomy(19) or any intestinal surgery which involves gastric resection, sleeve or banding surgery(20) Prolonged use of acidsuppression therapy or drugs(21) Alcoholism(22) MTHFR C677T polymorphism(23) Other gastrointestinal causes Chronic gastrointestinal symptoms e.g. dyspepsia, recurrent peptic ulcer, diarrhoea(3) Coeliac disease(24) Crohn’s disease(25) Tapeworms and other intestinal parasites(19) 1.1.5 Diseases caused by folate deficiency The main function of folate is to help form red blood cells (RBCs) and produce DNA. Folate also prevents neural tube defects (NTD) during fetal development.(26) A deficiency in folic acid can lead to increased homocysteine levels in plasma since folate is necessary for the conversion of homocysteine to methionine (see Figure 1.1).(3) In addition, deficiency can result in perturbation of the metabolic pathway of conversion of homocysteine to methionine with consequent disruption of DNA synthesis caused by thymidine lack, resulting in megaloblastic anaemia, as well as other adverse effects on the nervous system and other organs.(3) Table 1.2: Clinical manifestations of folate deficiency Haematologic (3) Megaloblastic anaemia Panycytopenia (Leukopenia, thrombocytopenia) Pernicious anaemia (i.e. large immature RBCs) Neurologic(27) Paresthesias (i.e. a skin sensation such as burning or itching with no apparent physical cause) Peripheral neuropathy Combined systems disease (demyelination of peripheral nerves, spinal cord, cranial nerves and the brain) Psychiatric(27-30) Irritability, personality change Mild memory impairment, dementia Depression Psychosis Alzheimer’s Disease(30) 1.1.6 Dietary sources of folate Folate is present in a variety of foods including leafy green vegetables, fruits and dried beans and peas. Examples of some of the dietary food sources and their folate content are also shown in Table 1.3. MBS Reviews – Folate Review Report Page 10 February 2014 Table 1.3: Examples of dietary sources of folate(31, 32) Type of food Lentils (1/2 cup cooked) Spinach (1/2 cup cooked) Tomato juice (1 cup) Orange juice (1 cup) Green peas (1/2 cup cooked) Strawberries Baked beans (1 cup) Banana (1 medium) Estimated folate content (micrograms) 179 115 49 47 47 40 30 24 Food fortification is the process of adding micronutrients (such as vitamins and minerals) to food as permitted by the Australian and New Zealand Food Standards Code (ANZFSC).(33) Regulations regarding the fortification of foods with folate vary between countries. The voluntary addition of folic acid to certain foods has been permitted in Australia since 1996.(34) Since then, a variety of products have been fortified with folic acid. However, the mandatory fortification of folate in Australia was initiated in September 2009 under Clause 4 (2) of Standard 2.1.1 of the ANZFSC. This ANZFSC states that folic acid (folic acid is the synthetic form of folate)(35) is added within the prescribed range of 200–300 μg per 100 grams of wheat flour used for bread-making.(36) This level of folic acid fortification was expected to prevent between 14 and 49 cases of NTD per year in Australia.(36) 1.1.7 Prevalence of folate deficiency in Australia The prevalence of folic acid deficiency in the general Australian population is unknown. A report published by the Australian Institute for Health and Welfare (AIHW) in 2011 found that the mean folic acid intake for women aged 16–44 years (the target population) in Australia before the introduction of the mandatory folic acid fortification program was 108 μg/day, which is well below the recommended 400 μg/day.(37) In addition, there were 149 pregnancies affected by NTD in 2005 in Australia (rate of 13.3 per 10,000 births) in the three states that provide the most accurate baseline of NTD incidence (South Australia, Western Australia and Victoria).(37) A retrospective study was conducted between April 2007 and April 2010 to determine the impact of the mandatory folic acid fortification program on the blood folate levels of an Australian population since its introduction in 2009.(38) This study reported that the prevalence of low serum folate levels decreased by 77% (from 9.3% to 2.1%) in all samples tested (the samples constituted 20,592 blood samples collected from a wide variety of patients and analysed in a public hospital diagnostic pathology laboratory). The prevalence of low RBC folate levels also decreased by 85% (from 3.4% to 0.5%).(38) The prevalence of low RBC folate levels for females of childbearing age was 0.16% of all samples. However, there were no data on the incidence of NTDs in newborn babies. A 31% increase in mean serum folate levels (from 881 nmol/L to 1071 nmol/L) was reported. The authors of this study concluded that the introduction of the mandatory fortification with folic acid has significantly reduced the prevalence of folate deficiency in Australia, including women of childbearing age.(38) 1.1.8 Folate testing MBS Reviews – Folate Review Report Page 11 February 2014 The following tests are commonly used for the detection of folate:(39) Serum folate: This is the usual first test for folate deficiency. Serum folate depends on recent dietary intake, therefore this test does not reflect the long-term folate status in the body. The normal reference range for serum folate is 7-45 nmol/L (3-20 ng/ml) (40). Red blood cell folate: This test measures the amount of folate in RBCs and is reflective of the long-term folate status in the body (i.e. stores in the liver).(41) However, this test is more complex to perform than the serum folate assay and requires more steps in sample handling before analysis, which may be one of the reasons why the precision of the red cell folate assay is less than that of the serum folate assay.(42) In addition, red cell folate concentrations are often low in patients with B12 deficiency(41) and are inversely associated with haemoglobin concentrations.(43) The reference interval for red cell folate is highly method dependent. An approximate normal range for red cell folate is 317-1422 nmol/L RBC (140-602 ng/ml RBC).(40) Levels less than 140 ng/mL are indicative of folate deficiency. Plasma homocysteine: This test is reflective of low or deficient folate status and is therefore considered an indicator of folate or vitamin B12 adequacy. However, plasma homocysteine levels are elevated in patients with renal failure(44) and with ageing(45, 46). Special care (and collection tubes) should be taken when collecting blood samples for testing using this method as homocysteine concentration can rise after blood collection in certain tubes due to ongoing release of homocysteine by the RBCs in vitro.(47) There is a lack of strong evidence in the literature to support the clinical practice of performing serum folate as well as RBC folate measurements in an individual to detect folate deficiency. The evidence that suggests that red cell folate is a better indicator of folate status than serum folate is old(48) and the methodology used is less reliable than modern-day assays used for the assessment of serum folate. A more recent study that analysed the use of the two methodologies in determining folate deficiency in individuals reported that serum folate measurements provide equivalent information to RBC folate measurements.(49) The alternative approach for assessment of folate status involves measurement of the metabolite homocysteine, which is known to increase in folate deficiency and provides certain advantages over direct measurement of serum folate concentrations.(4, 50) However, homocysteine requires both folate and vitamin B12 for its conversion to methionine (refer to Figure 1.1). Consequently, plasma homocysteine concentrations rise in both folate and vitamin B12 deficiencies. Therefore, the only way to distinguish whether an individual is deficient in either of these vitamins is to measure serum MMA. Increased serum levels of MMA are solely attributed to vitamin B12 deficiency. It is important to note that the correction of folate deficiency through the use of supplementation and/or fortification may mask an occult vitamin B12 deficiency and further exacerbate or initiate neurologic disease. Therefore, it has been recommended that clinicians consider ruling out vitamin B12 deficiency before initiating folic acid therapy.(51) 1.1.9 Folate reference ranges The cut-off value for folate deficiency varies markedly between laboratories worldwide. Table 1.4 presents the ‘usual or approximate’ reference intervals for folate deficiency. MBS Reviews – Folate Review Report Page 12 February 2014 Table 1.4: Folate (40) reference intervals Status Normal range Deficient Serum folate (nmol/L) 7-40 <7 RBC folate (nmol/L) 360-1400 < 360 1.1.10 MBS item for folate testing The MBS item numbers for folate testing in scope of this review include 66599 and 66602 (see Appendix 3). Both of the items relate to testing serum vitamin B12 or testing red cell (or serum) folate. Both of the items are subject to Rule 21 (i.e. no more than three of any combination of these tests are eligible for Medicare subsidy per patient per year). There is no MBS item for folate testing on its own. 1.1.11 Service providers claiming MBS benefits for folate testing Most pathology in Australia is provided in comprehensive laboratories that provide a wide range of testing services at a single location. Only approved pathology practitioners are eligible to claim folate testing. 1.2 The clinical flowcharts The clinical decision pathway that determines whether folate testing should be undertaken is provided in Figure 1.2. MBS Reviews – Folate Review Report Page 13 February 2014 Figure 1.2: Clinical flow chart for folate testing Patient presents to clinician (e.g. General Practitioner, Obstetrician etc) Does the patient have any of the following clinical symptoms of folate deficiency? Neuropsychiatric symptoms (mild) including: • dementia; and/or • depression; and/or • Psychosis; and/or • personality changes. Does the patient have any of the following haematological symptoms of folate deficiency? Does the patient have any of the following risk factors associated with folate deficiency? • • • • • • anaemia; and/or • macrocytosis. No Yes Patient ineligible to claim benefits under MBS item numbers 66599 or 66602 Is Vitamin B12/folate testing clinically relevant? No MBS Reviews – Folate Review Report patients with coeliac disease; and/or pregnancy; and/or dietary deficiency; and/or alcoholism; and/or malignancy (e.g. leukaemia). Page 14 Yes Measure Vitamin B12 and/or folate and claim MBS item 66602 or 66599 February 2014 2 REVIEW METHODOLOGY The review methodology comprises an analysis of secondary data (MBS claims), a guideline concordance analysis, and a systematic literature review for clinical and economic evidence. This Chapter presents clinical research questions and the methodology used for each of these review components. 2.1 Secondary data analysis Data from Medicare Australia were analysed to determine whether the existing MBS item numbers for folate testing (66599 and 66602) are appropriate. 2.1.1 The research questions for the MBS analysis The MBS data were examined to determine: (1) Whether the existing MBS items for service (66599 and 66602), including the associated explanatory notes, are appropriate a. How frequent are the MBS item numbers under review claimed? b. Are there any age, sex, temporal or geographic trends associated with usage of these item numbers? c. Are the Medicare claims data consistent with trends in the incidence/prevalence of the conditions/diseases being addressed by the services? d. What is the frequency of folate testing per patient? e. What is the frequency of folate testing by referring clinician? f. What is the profile of referring clinician for folate testing? 2.1.2 Methods for analysis of MBS data MBS data relates to private medical services (provided in- or out-of-hospital), where the services are provided to patients regardless of whether or not they have private health cover. MBS in-hospital services are mainly provided in private hospitals and day surgery clinics, but patients can elect to be treated as a private patient in a public hospital. MBS data were analysed by patient gender, age group, and discipline of provider requesting the service. Results of the analysis of the MBS data is presented in Chapter 3. 2.2 Guideline concordance 2.2.1 The research questions for the guideline concordance analysis The research question addressed as part of the Review using guideline concordance analysis are: (1) Are the existing MBS items for service consistent with evidence-based (or in the absence of evidence, consensus-based) recommendations provided in relevant clinical practice guidelines? (2) What are the appropriate clinical indications for folate testing? MBS Reviews – Folate Review Report Page 15 February 2014 (3) How frequently should folate levels be tested? a. in apparently healthy populations (including pregnant women, elderly, vegetarians)? b. in patients with chronic disease linked to folate deficiency (e.g. infants with metabolic disease; patients with anaemia or haematologic , neurologic, psychiatric, gastrointestinal and malabsorption disorders)? 2.2.2 Methods for guideline concordance analysis Searches of guidelines databases1 and relevant discipline websites were undertaken to locate any existing guidelines relevant to folate testing. Analysis of MBS item numbers 66599 and 66602 was undertaken relative to ‘best practice’, as recommended in relevant Australian clinical practice guidelines. Where Australian clinical practice guidelines do not exist, other guidelines in operation in comparable health systems overseas were included. Where guidelines existed, they were assessed for quality using the AGREE II instrument(52). Differences in the purpose and intended audience of any such guidelines were considered, documented and acknowledged. See Chapter 4 for results of the concordance analysis for folate testing. 2.3 Systematic literature review for clinical evidence 2.3.1 The clinical/research questions for the systematic literature review The clinical/research questions that were the focus of the literature review are: (1) What are the appropriate clinical indications for folate testing? (2) Is there evidence that testing for folate levels improves health outcomes? a. in apparently healthy populations (including pregnant women, elderly, vegetarians)? b. in patients with chronic disease linked to folate deficiency? (3) Are there risks/harms associated with folate testing? (4) Does quality of testing vary according to testing platform? 2.3.2 Search strategy A comprehensive search of peer-reviewed scientific literature was conducted to identify relevant studies addressing the key questions. Electronic databases were searched for original research papers including systematic reviews as shown in Table 2.1. Searches were restricted to studies published in the English language between January 2002 and July 2013. Databases maintained by Health Technology Assessment (HTA) agencies were searched to identify existing assessments of folate testing. 1 The search included: Guidelines International Network (G-I-N) at www.g-i-n.net/library/internationalguidelines-library/; National Guidelines Clearinghouse at www.guidelines.gov; National Health and Medical Research Council (NHMRC) at http://www.nhmrc.gov.au/guidelines-publications MBS Reviews – Folate Review Report Page 16 February 2014 Table 2.1: Databases searched Database MEDLINE PubMed The Cochrane Library (includes Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, Cochrane Central Register of Controlled Trials, NHS Economic Evaluation Database, Health Technology Assessment, Cochrane Methodology Register) Relevant HTA websites and databases2 Search period 2002 – September 2013 2002 – September 2013 2002 – September 2013 Up to September 2013 Reference lists of systematic, semi-systematic and selected narrative reviews were also reviewed. In addition, during the consultation process clinicians were asked if they were aware of any relevant clinical guidelines, unpublished studies or reviews relevant to this review of folate testing. 2.3.3 Eligibility criteria for studies The PICO (Population, Intervention, Comparator, Outcomes) criteria(53) was used to develop well-defined questions for the search of published literature. This involved focusing the question on four elements: the target population for the intervention; the intervention being considered; the comparator for the existing MBS service (where relevant); and the clinical outcomes that are most relevant to assess safety and effectiveness. The PICO criteria were determined on the basis of information provided in the literature, as well as clinical advice. The PICO criteria for the review of folate testing is shown in Table 2.2. Table 2.2: PICO criteria for the folate testing items under review Population Intervention Comparator Outcomes Patients at risk of folate deficiency, Effectiveness including (but not limited to): Physical health outcomes (1) Pregnant women as a consequence of folate (2) Elderly testing (3) Alcoholics Supplementation Safety (4) Patients with gastrointestinal Folate testing without testing Complications associated and malabsorption disorders with folate testing (e.g. (5) Patients with anaemia and infection, needle injuries) haematologic diseases The detailed search strategy and terms used is presented in Appendix 4. 2 The following HTA websites were searched: Agency for Healthcare Research and Quality (AHRQ) at www.ahrq.gov; Canadian Agency for Drugs and Technologies in Health (CADTH) at http://www.cadth.ca/en; National Institute for Health and Care Excellence (NICE) at www.nice.org.uk; Australasian College of Surgeons (ASERNIP-S) at http://www.surgeons.org/for-health-professionals/audits-and-surgical-research/asernip-s/ MBS Reviews – Folate Review Report Page 17 February 2014 Studies were excluded on the basis of citation information and/or abstract, where it was obvious that they did not meet the inclusion criteria. Where there was any doubt about any reference based on the title and/or abstract, the full paper was retrieved and evaluated. Table 2.3 lists the pre-specified inclusion and exclusion criteria. Table 2.3: Inclusion/exclusion criteria for identification of relevant studies Characteristic Criteria Search period 2002 – 2013 Should there be limited data available during this period, the search will be extended back in five year increments until sufficient data are sourced. Clinical studies included. Non-systematic reviews, letters, editorials, animal, in vitro Publication and laboratory studies excluded. type Systematic reviews Systematic reviews that have been superseded were excluded Primary studies Primary studies published during the search period of included systematic reviews were excluded Effectiveness studies included if: prospective, comparative trial >20 patients Safety studies included if: >50 patients Intervention Folate testing Comparator Supplementation without folate testing Studies must report on at least one of the following outcomes: Outcome Patient outcomes: morbidity, mortality, quality of life Safety: adverse physical health outcomes or complications associated with testing Non-English language articles excluded Language 2.3.4 Process for classifying the evidence All eligible studies were assessed according to the National Health and Medical Research Council (NHMRC) Dimensions of Evidence (refer to Appendix 5). There are three main domains: strength of the evidence, size of the effect, and relevance of the evidence. One aspect of the ‘strength of the evidence’ domain is the level of evidence, which is assigned using the NHMRC Levels of Evidence (Appendix 5). For any eligible publications, study quality was evaluated and reported using the NHMRC Quality Criteria (Appendix 5) for randomised controlled trials (RCTs), cohort studies, case-control studies and systematic reviews. The results of the review of clinical evidence for folate testing are presented in Chapter 5. 2.4 Systematic literature review for economic evidence The research question for the review of economic literature is: (1) What is the evidence regarding the cost implications associated with folate testing compared with not testing Consistent with the terms of reference, a formal modelled economic evaluation of folate testing was not in scope. The review relied on published costing studies and economic MBS Reviews – Folate Review Report Page 18 February 2014 analyses identified through a systematic literature search of the databases shown in Table 2.1. The detailed search strategy and terms used is presented in Appendix 4. Citations were reviewed to identify acceptable evidence including: trial-based costing studies, cost analyses and economic modelling studies. Acceptable outcomes were limited to: cost, incremental cost-effectiveness ratio (e.g. cost per event avoided, cost per life year gained, cost per quality adjusted life year or disability adjusted life year). The results of the search for economic evaluations of folate testing are presented in Chapter 6. MBS Reviews – Folate Review Report Page 19 February 2014 3 SECONDARY DATA ANALYSIS This Chapter presents an analysis of the available secondary data (including MBS data) that describes the use of folate testing in Australia. When interpreting the data, it is important to keep in mind that the MBS item numbers within scope are for both folate and vitamin B12 testing. It is not possible to separate the data that specifically relates to folate testing alone. 3.1 MBS item number usage and expenditure Figure 3.1 shows the number of claims for each of the MBS folate/vitamin B12 testing items over the past 10 years. The number of claims for MBS item 66599 has more than doubled (+119%) from 282,531 in 2003/04 to 618,744 in 2012/13. Over the same timeframe the number of claims for MBS item 66602 has had an even greater increase (+307%) from 522,980 to 2,129,051. The total number of claims for both items has increased over the past ten years from 0.98m to 2.7m. Figure 3.1: Number of claims for MBS items 66599 and 66602, 2003/04 to 2012/13 Number of MBS item claims 2,500,000 2,000,000 1,500,000 1,000,000 500,000 0 66602 66599 Source: Department of Human Services – Medicare Australia Figure 3.2 shows the benefits paid for MBS items 66602 and 66599 over the past ten years by state. The increase in benefits paid for both items reflects the increase in claims. Benefits paid for MBS item 66599 increased from $5.7m to $12.5m (+120%) whilst benefits for MBS item 66602 increased from $19.2m to $78.5m (+309%). Whilst total benefits increased significantly, the proportion of benefits paid to each state and territory remained relatively constant over the ten-year period. MBS Reviews – Folate Review Report Page 20 February 2014 Figure 3.2: Benefits paid for MBS items 66599 and 66602 by state and territory, 2003/04 to 2012/13 Medicare benefits paid Item 66599 $12,000,000 $10,000,000 $8,000,000 $6,000,000 $4,000,000 $2,000,000 $0 2003/2004 2004/2005 2005/2006 2006/2007 2007/2008 2008/2009 2009/2010 2010/2011 2011/2012 NSW VIC SA QLD WA TAS ACT NT Item 66602 Medicare benefits paid $90,000,000 $80,000,000 $70,000,000 $60,000,000 $50,000,000 $40,000,000 $30,000,000 $20,000,000 $10,000,000 $0 2003/2004 2004/2005 2005/2006 2006/2007 2007/2008 2008/2009 2009/2010 2010/2011 2011/2012 2012/2013 NSW VIC QLD SA WA TAS ACT NT Source: Department of Human Services – Medicare Australia Figure 3.3 shows that the highest proportion of benefits paid over the past ten years was in New South Wales (NSW) (34% for 66599 and 38% of total for 66602). This was followed by Victoria (30% of total for 66599 and 28% of total for 66602). For MBS item 66602, Queensland had the third highest proportion of claims (19%) followed by Western Australia (8%) and South Australia (5%). This pattern was slightly different for MBS item 66599, with South Australia having the third highest proportion of claims (13%) followed by Queensland (11%) and Western Australia (8%). MBS Reviews – Folate Review Report Page 21 February 2014 Figure 3.3: Proportion of total benefits paid for MBS items 66599 and 66602 by state and territory, July 2003 to June 2013 Percent of benefits paid 40% 35% 30% 25% 20% 15% 10% 5% 0% NSW VIC QLD SA 66602 WA TAS ACT NT 66599 Source: Department of Human Services – Medicare Australia To further explore geographical trends in testing, an analysis was conducted of the number of services per capita (i.e. per 100,000 population), according to the address at the time of claiming of the patient to whom the service was rendered. In 2012/13, there were 2,666 claims per 100,000 people enrolled in Medicare across Australia for item 66599 and 9,172 claims per 100,000 people for item 66602 (Table 3.1). South Australia had the highest rate of claiming for item 66599 per capita (3,635 claims per 100,000 population), followed by the Australian Capital Territory (ACT) and NSW. The lowest per capita rate of claims for item 66599 was in the Northern Territory (762 claims per 100,000 population). For item 66602, the highest number of claims per capita in 2012/13 was for NSW and Victoria (over 10,000 claims per 100,000 population in both states), while Tasmania had the lowest (less than 4,000 claims per 100,000 population). Table 3.1: Claims for MBS items 66599 and 66602 per capita (100,000 population)*, 2008/09 to 2012/13 State/ territory NSW VIC QLD SA WA TAS ACT NT Total 2008/09 1,922 1,928 1,013 3,034 1,525 1,910 2,163 420 1,773 Item 2009/10 2,065 2,052 961 3,172 1,165 2,204 2,285 555 1,822 66599 2010/11 2,343 2,155 1,195 3,094 1,329 2,435 2,310 676 2,002 2011/12 2,635 2,472 1,608 3,327 1,387 2,522 2,602 703 2,286 2012/13 3,021 2,918 2,050 3,635 1,599 2,732 3,046 762 2,666 2008/09 7,774 7,860 6,174 4,895 5,025 3,796 8,831 1,698 6,849 Item 2009/10 8,299 8,454 6,157 5,015 5,778 3,630 8,658 1,962 7,243 66602 2010/11 8,561 8,435 6,787 4,994 5,987 3,462 8,191 2,329 7,462 2011/12 9,134 8,985 7,589 5,570 6,021 3,546 8,256 2,796 7,998 2012/13 10,401 10,261 8,822 6,483 6,887 3,947 9,265 4,639 9,172 Source: Department of Human Services – Medicare Australia * Services per capita (i.e. per 100,000 population) is calculated by dividing the number of services processed in a month by the number of people enrolled in Medicare at the end of that month. Data relating to the average fee per service and average benefit per service from 2008/09 to 2012/13 are summarised in Table 3.2. The proportion of services bulk billed was high (more than 94% of services) from 2008/09 to 2012/13, which is consistent with the high proportion of out-of-hospital services (approximately 97%). MBS Reviews – Folate Review Report Page 22 February 2014 Table 3.2: Fees charged and benefits paid for MBS items 66599 and 66602, 2008/09 to 2012/13 Total number of services Total fees charged Average fee per service Total benefits paid Average benefit per service Out-of-hospital services Services bulkbilled Average OOP cost* Item 66599 66602 66599 66602 66599 66602 66599 66602 66599 66602 66599 66602 66599 66602 66599 66602 2008/09 382,241 1,476,465 $7,995,258 $56,002,248 $20.91 $37.93 $7,843,976 $55,208,644 $20.52 $37.39 96.8% 97.5% 94.2% 95.3% $9.14 $16.65 2009/10 399,282 1,586,968 $8,310,784 $60,132,026 $20.81 $37.89 $8,102,717 $58,728,189 $20.29 $37.00 97.3% 97.4% 94.3% 93.8% $12.33 $18.17 2010/11 447,211 1,667,155 $9,225,951 $62,715,325 $20.63 $37.62 $9,061,322 $61,669,203 $20.26 $36.99 97.3% 97.0% 95.8% 95.4% $13.00 $20.44 2011/12 520,688 1,821,490 $10,698,027 $68,264,085 $20.54 $37.48 $10,545,169 $67,358,102 $20.25 $36.97 97.4% 97.2% 96.0% 95.7% $9.92 $15.54 2012/13 618,744 2,129,051 $12,665,119 $79,337,023 $20.46 $37.26 $12,484,776 $78,506,111 $20.17 $36.87 97.1% 97.4% 96.2% 96.3% $10.26 $14.34 Total 2,368,166 8,681,129 $48,895,138 $326,450,707 $20.64 $37.60 $48,037,960 $321,470,248 $20.28 $37.03 97.2% 97.3% 95.4% 95.4% $10.96 $17.29 Source: Department of Human Services – Medicare Australia *Average out-of-pocket cost is equal to ‘fees charged for patient-billed out-of-hospital services’ minus ‘benefits paid for patient-billed outof-hospital services’ divided by ‘number of patient-billed out-of-hospital services’ 3.2 Age and gender profile of patients The pattern of use by age and gender for item numbers 66599 and 66602 is shown in Figure 3.4. Folate/vitamin B12 testing claimed under MBS item numbers 66599 and 66602 is performed for both males and females; however, on average (across all age groups), the test is undertaken more frequently in females than males (1.8 times more frequently for MBS item 66599 and 1.9 times more frequently for MBS item 66602). For both MBS items, this difference is greatest in the 15-24 and 25-34 year age groups where females are over 3 times more likely to have a folate/vitamin B12 test. For both males and females, the number of tests being performed is higher in older age groups, particularly for MBS item 66599. This trend was slightly greater for males, with 76% of claims for MBS item 66599 being for those aged 45 years and over versus 64% for females. Similarly, for MBS item 66602, 71% of claims for males were aged 45 years and over versus 60% for females. MBS Reviews – Folate Review Report Page 23 February 2014 Figure 3.4: Use of the MBS items for B12/folate testing by age and gender (2008/09 to 2012/13) 300,000 Number of services for MBS item 66599 Number of services for MBS item 66602 1,000,000 900,000 800,000 700,000 600,000 500,000 400,000 300,000 200,000 100,000 0 250,000 200,000 150,000 100,000 50,000 0 Female Male Female Male Source: Department of Human Services – Medicare Australia 3.3 Frequency of testing by patient An analysis of folate/vitamin B12 testing frequency per patient was conducted. For item 66599, there was an increase3 in the overall number of patients tested for folate/vitamin B12, from 350,954 patients in 2008/09 to 502,547 patients in 2012/13 (+43%). As can be clearly seen from Figure 3.5, over the period 2008/09 to 2012/13 there has been very little change in the proportion of patients receiving either one test per year (92%), two tests per year (7.5%), or three or more tests per year (0.8%). For item 66602, there was an increase in the overall number of patients tested for folate/vitamin B12, from 1,324,646 in 2008/09 to 1,706,258 in 2012/13 (+29%). The proportion of patients receiving only one test within a year was stable over the five-year timeframe (90%). Approximately 9% of patients received two tests per year and approximately 1% received three or more tests per year. These data suggest that the majority of folate/vitamin B12 testing services are being undertaken for the purposes of screening/testing rather than monitoring. 3 Based on data processed to 31 May 2013; 2012/13 is therefore incomplete. MBS Reviews – Folate Review Report Page 24 February 2014 Figure 3.5: Frequency of claiming MBS items 66599 and 66602 per year by patient, 2008/09 to 2012/13* Percentage of patients Item 66602 100.0% 80.0% 60.0% 40.0% 20.0% 0.0% 2008/09 2009/10 1 test 2010/11 2 tests 2011/12 2012/13 2011/12 2012/13 3+ tests Percentage of patients Item 66599 100.0% 80.0% 60.0% 40.0% 20.0% 0.0% 2008/09 2009/10 1 test 2010/11 2 tests 3+ tests Source: Department of Human Services – Medicare Australia * Based on data processed to 31 May 2013; 2012/13 is therefore incomplete. 3.4 Profile of providers requesting folate/vitamin B12 testing services The profile of providers requesting folate/vitamin B12 services was examined over time from 2008/09 to 2012/13 (Table 3.3). Over the five-year time period, there were no material changes in the pattern of requesting providers. General practitioners (GPs) and other medical practitioners (OMPs) accounted for approximately 71% of all providers requesting folate/vitamin B12 testing services for item 66599. Internal medicine consultant physicians accounted for approximately 14% of all provider counts over the five-year time period, followed by general surgeons (specialist – subspecialties) and interns. There was a large variety of other providers requesting services, but they each accounted for less than 2% of provider counts. The profile of providers requesting item 66602 was similar to that of item 66599. GPs and OMPs accounted for 67% of all providers requesting item 66602, followed by internal medicine consultant physicians (approximately 14%), psychiatrists, general surgeons (specialist – subspecialties) and interns. MBS Reviews – Folate Review Report Page 25 February 2014 Table 3.3: Number of providers requesting MBS items 66599 and 66602, 2008/09 to 2012/13 Providers requesting folate/vitamin B12 testing services Item 66599 GPs and OMPs Consultant physician - internal medicine General Surgeon - Specialist subspecialties Intern Psychiatrist Obstetrics & Gynaecology (Specialist & Non-Specialist) General Surgeon - Specialist Other Temporary Resident Doctors All other provider groups# Item 66602 GPs and OMPs Consultant physician - internal medicine Psychiatrist General Surgeon - Specialist subspecialties Intern General Surgeon - Specialist Obstetrics & Gynaecology (Specialist & Non-Specialist) Other Temporary Resident Doctors All other provider groups# 2008/09€ 2009/10€ Provider 2010/11 count 2011/12 2012/13* % of all providers July 2008 to May 2013 19,882 3,859 20,363 3,955 21,533 4,283 23,263 4,884 24,019 5,135 70.9% 14.4% 648 600 677 819 759 2.3% 491 494 488 574 493 495 529 567 550 873 705 635 885 703 643 2.2% 1.9% 1.8% 414 211 1,016 427 228 1,042 454 243 1,172 525 439 1,428 527 476 1,490 1.5% 1.0% 4.0% 23,424 4,723 24,403 5,184 25,554 5,454 26,802 5,744 27,843 5,834 67.3% 14.2% 1,090 1,075 1,169 1,193 1,272 1,251 1,371 1,307 1,374 1,305 3.3% 3.2% 790 625 586 904 666 592 938 689 612 1,056 711 662 918 720 643 2.4% 1.8% 1.6% 398 1,649 452 1,769 493 1,953 503 2,076 426 2,102 1.2% 5.0% Source: Department of Human Services – Medicare Australia € Data are incomplete for 2008/09 and 2009/10 because small provider counts were suppressed. * Based on data processed to 31 May 2013; 2012/13 is therefore incomplete. # Other includes the following peer groups, which each accounted for <1% of the provider count: Dermatologist – specialist; Surgeon – nonspecialist; Other medical specialist; IVF; Unclassified-miscellaneous-non-specialist; Anaesthetics – specialist; Dentist/orthodontist; Therapeutic radiologist/therapeutic nuclear medicine – specialist; Pathologist; Anaesthetics – non-specialist; Specialist physician – internal medicine; Diagnostic imagist – specialist; Diagnostic imagist – non-specialist; Acupuncture; Other, other allied health, abortion/fertility control; Unclassified-miscellaneous-specialist. 3.5 Frequency of requests for testing by provider An analysis was conducted of requests for item 66599 and 66602 by frequency from any one provider. For both items, there was an increase over the period 2008/09 to 2012/13 in the overall number of providers requesting folate/vitamin B12 testing. For item 66599, the majority of providers (97%) requested 100 or fewer tests per year. Approximately 2% of providers requested 101 to 200 tests, and the remaining providers requested more than 200 tests per year (Table 3.4). There were a small number of providers (ranging from 36 to 62 per year) that requested more than 400 tests per year. From 2008/09 to 2012/13, approximately 88% of the providers requesting item 66602 requested 100 or fewer tests per year (Table 3.4). Approximately 7% of providers requested between 101 and 200 tests per year and 2.5% requested 201 to 300 tests per year. Between 2010/11 and 2012/13, over 500 providers requested more than 400 tests per year. MBS Reviews – Folate Review Report Page 26 February 2014 Table 3.4: Frequency of MBS items 66599 and 66602 requested per year by any provider, 2008/09 to 2012/13* Provider count per test number category (% of providers) No. of tests requested in the year Item 66599 1 – 100 101 – 200 201 – 300 301 – 400 401 + Total Item 66602 1 – 100 101 – 200 201 – 300 301 – 400 401 + Total 2012/13* 2008/09 2009/10 2010/11 2011/12 Total 26,884 (97.7%) 458 (1.7%) 120 (0.4%) 24 (0.1%) 42 (0.2%) 27,528 (100%) 27,521 (97.6%) 469 (1.7%) 123 (0.4%) 46 (0.2%) 36 (0.1%) 28,195 (100%) 29,205 (97.3%) 559 (1.9%) 133 (0.4%) 56 (0.2%) 55 (0.2%) 30,008 (100%) 32,600 (97.1%) 678 (2.0%) 172 (0.5%) 59 (0.2%) 62 (0.2%) 33,571 (100%) 33,602 (97.0%) 149,812 (97.2%) 724 (2.1%) 2,888 (1.9%) 185 (0.5%) 733 (0.5%) 66 (0.2%) 251 (0.2%) 60 (0.2%) 255 (0.2%) 34,637 (100%) 153,939 (100%) 30,487 (88.7%) 2,260 (6.6%) 772 (2.2%) 380 (1.1%) 461 (1.3%) 34,360 (100%) 32,203 (88.6%) 2,390 (6.6%) 869 (2.4%) 384 (1.1%) 486 (1.3%) 36,332 (100%) 33,819 (88.5%) 2,527 (6.6%) 912 (2.4%) 438 (1.1%) 520 (1.4%) 38,216 (100%) 35,402 (88.0%) 2,706 (6.7%) 1,032 (2.6%) 510 (1.3%) 582 (1.4%) 40,232 (100%) 35,985 (87.4%) 167,896 (88.2%) 2,917 (7.1%) 12,800 (6.7%) 1,152 (2.8%) 4,737 (2.5%) 570 (1.4%) 2,282 (1.2%) 541 (1.3%) 2,590 (1.4%) 41,165 (100%) 190,305 (100%) Source: Department of Human Services – Medicare Australia * Based on data processed to 31 May 2013; 2012/13 is therefore incomplete. MBS Reviews – Folate Review Report Page 27 February 2014 4 REVIEW OF GUIDELINES RELEVANT TO FOLATE TESTING This Chapter presents the results of the literature search for clinical practice guidelines and the guideline concordance analysis conducted for folate testing. 4.1 Australian guidelines The MBS data (Chapter 3) indicate that the majority of requests for folate/vitamin B12 testing are initiated by GPs and OMPs. The relevant College proving practice advice is the Royal Australian College of General Practitioners (RACGP). In 2012 the Royal Australian College of General Practitioners (RACPG) published guidelines for preventative activities in general practice.(54) The ‘red book’ has been published since 1989 and is accepted as the main guide to preventative care in Australian general practice. The intention is to provide a comprehensive and concise set of recommendations for patients in general practice. The recommendations in the guidelines are based on current, evidence-based guidelines for preventative activities relevant to Australian general practice. Where Australian guidelines are not available or recent, other sources have been used, such as Canadian or United States preventative guidelines or the results of systematic reviews. Folate supplementation is only mentioned in one section of the RACGP guidelines. In the section on pre-conception preventative interventions, folate supplementation is recommended. The guidelines do not comment on testing for folate levels, which indicates that it is not necessary for this population. In March 2013, the Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG) updated their College Statement (C-Obs 25) on vitamin and mineral supplementation and pregnancy. The statement makes recommendations for folate supplementation, without any mention of the need for measurement of folate levels. The College recommends that folic acid should be taken for a minimum of one month before conception and for the first 12 weeks of pregnancy. The recommended dose of folic acid is increased where there is an increased risk of NTD (e.g. anticonvulsant medication, prepregnancy diabetes mellitus, previous child or family history of NTD or BMI >30). Women at increased risk of folate deficiency (e.g. multiple pregnancies, haemolytic anaemia) are advised to take 5 mg of folic acid throughout the pregnancy. No Australian clinical practice guidelines were identified that specifically mention folate testing. 4.2 International guidelines Although there are a large number of international clinical practice guidelines that recommend folate supplementation in specific populations, in most cases they do not mention or make explicit recommendations regarding folate testing. The implication is that supplementation should commence without measurement of folate levels. The following guidelines are those that were identified that specifically mention folate testing. Although somewhat dated, a best practice review was published by Smellie et al. (2005) on the diagnosis and monitoring of vitamin B12 (and folate) deficiency.(55) The review was MBS Reviews – Folate Review Report Page 28 February 2014 based on a standardised literature search of national and international guidance notes, consensus statements, health policy documents, and evidence based medicine reviews, supplemented by relevant primary research documents. However, the authors state that the recommendations were mostly ‘consensus rather than evidence based’. Therefore, the guidance was derived from a small number of reviews, supplemented by extrapolations from knowledge of the physiology of vitamin B12 and folate. The review recommended that folate testing (together with vitamin B12 testing) should be performed for the following indications: macrocytic anaemia; macrocytosis; and specific neuropsychiatric abnormalities (including paraesthesia, ataxia, peripheral neuropathy, and memory loss). In terms of monitoring in patients who have or are receiving replacement, the authors state that there is no obvious merit in repeating folate measurements during replacement unless lack of compliance is suspected or anaemia recurs. There are several disease-specific guidelines that mention the measurement of folate levels. The National Institute for Health and Clinical Excellence (NICE) in the United Kingdom released guidance in November 2006 (modified October 2012) to support people with dementia and their carers in health and social care.(56) The guideline recommends that measurement of serum vitamin B12 and folate levels are included in a basic dementia screen, which is performed at the time of presentation (usually within primary care). In 2007, the UK Royal College of General Practitioners released guidance on the diagnosis and management of chronic fatigue syndrome/myalgic encephalomyelitis (or encephalopathy) in adults and children.(57) The guidance states that tests for vitamin B12 deficiency and folate levels should not be carried out unless a full blood count and mean cell volume show a macrocytosis. 4.3 Other reports In July 2013, Health Quality Ontario released recommendations on folate testing from the Appropriateness Working Group of the Ontario Health Technology Advisory Committee (OHTAC)(58). The objective of their Appropriateness Initiative is to develop a systematic framework for the ongoing identification, prioritisation, and assessment of health interventions in Ontario for which there is possible misuse, overuse, or underuse. Seven health interventions were examined in the first phase: annual health exams, aspartate aminotransferase testing, ferritin testing, folate testing and vitamin B12 testing. Due to the very limited evidence base, the analysis of folate testing was undertaken using expert consultation and consensus rather than rapid reviews or full evidence-based analyses.(59) In the background section, the document states that the five specialties (in Ontario) that ordered the most folate testing in the community were family practice/general practice (82%), internal medicine (4.5%), nurse practitioners (2.4%), neurology (2.4%), and gastroenterology (1.8%). Folate testing was used in males and females in all age categories; however, greatest use was in women. The provider profile for folate test requests and patient age and gender pattern was not dissimilar to Australia. Expert consultation identified health conditions where folate deficiency may be of concern and where folate testing may be appropriate. Based on the expert consultation, OHTAC recommended that red blood cell folate testing be restricted to individuals with: MBS Reviews – Folate Review Report Page 29 February 2014 low haemoglobin levels and a high mean corpuscular volume; and suspected gastrointestinal disorders causing malabsorption or suspected malnutrition of any cause. MBS Reviews – Folate Review Report Page 30 February 2014 5 REVIEW OF THE CLINICAL EVIDENCE FOR FOLATE TESTING This Chapter presents the results of the systematic literature review on folate testing in relation to the clinical research questions. 5.1 Evidence base 5.1.1 Search results A literature search was performed on 23rd September 2013, using OVID MEDLINE, EMBASE, and the Cochrane Library, for studies published from January 2002 until September 2013. Abstracts were reviewed and for those studies meeting the eligibility criteria, full-text articles were obtained. Reference lists were also examined for any additional relevant studies not identified through the search. The database search yielded 985 citations (with duplicates removed). Articles were excluded based on information in the title and abstract and the full texts of potentially relevant articles were obtained for further assessment. 5.1.2 Existing health technology reports and systematic reviews There were no health technology reports or systematic reviews on the safety and effectiveness of folate testing. One systematic review of poor quality was identified, which aimed to compare measurement of serum versus red cell folate.(60) 5.2 Appropriate clinical indications for folate testing There were no studies meeting the eligibility criteria that evaluated the clinical indications for folate testing. However, as discussed in Chapter 4, a limited number of clinical practice guidelines mention measurement of serum folate levels for particular populations. 5.3 Evidence that testing folate levels improves health outcomes No definitive conclusions can be drawn about the effectiveness of folate testing since no prospective trials have been conducted to directly assess the impact of testing on health outcomes in healthy populations or in patients with chronic disease associated with folate deficiency. Although retrospective studies do not meet the inclusion criteria for this review, several retrospective studies (low level evidence) were identified that examined the clinical utility of folate testing. For example, a retrospective study undertaken in the United States by Ashraf et al. (2008) examined the utility of folate testing for patients with anemia or dementia. During the 4-month study period, 1,007 folate tests were performed at their medical centre on 980 patients. Fourteen patients (0.4%) had folate levels that were low or borderline (four patients had levels < 3.0 ng/mL and ten patients had levels 3-4 ng/mL). All 14 patients were inpatients. Five patients received folate replacement and six patients had their folate levels rechecked within 12 months, at which time all levels had returned to normal. The authors concluded that their study demonstrates the low utility of routine folate testing in clinical practice; the test should be reserved for patients with macrocytic anaemia and those at high risk for folate deficiency.(61) MBS Reviews – Folate Review Report Page 31 February 2014 Another retrospective study from the United States analysed all inpatient and emergency department serum folate tests at a single institution over a twelve-month period to determine the indications, comorbidities and change in management based on the test results.(62) A total of 2,093 serum folate tests were performed in 1,944 patients, with only two patients (0.1%) having deficient levels (≤ 3.0 ng/mL). The most common indications for testing (based on a random sample of 250 chart reviews) were anaemia without macrocytosis, anaemia with macrocytosis, delirium, malnutrition, and peripheral neuropathy. Given the low rate of serum folate deficiency and the lack of change in management based on deficient results, the authors concluded that in folic acid fortified countries, serum folate testing has low utility for all indications in inpatients and emergency department patients.(62) The authors commented that based on prior studies, and supported by their results, there is no evidence for checking serum folate levels in delirium, dementia, peripheral neuropathy, malnutrition, or any of the other indications. In addition, their results demonstrated a low utility even in patients with anaemia or macrocytic anaemia. 5.4 Risks/harms associated with folate testing No trials designed to directly measure the risks or harms associated with folate testing were identified. However, folate testing relies on a blood draw, which is a safe procedure. It is likely that the consequences of inaccurate or inappropriately interpreted folate test results, such as a false positive, are relatively small. Folate supplements are generally considered safe when taken in amounts that are not higher than the recommended dietary allowance. 5.5 Quality of testing A poor quality systematic review by Farrell et al. (2012) sought to compare the effectiveness of serum versus red cell folate.(60) The authors undertook a literature search on Medline using the terms ‘red cell folate’ and ‘serum folate’ and identified 328 papers. A narrative assessment of the body of evidence was undertaken. The authors declared no conflicts of interest. After assessing different aspects of the performance of serum and red cell folate assays, the review did not find evidence to justify the higher cost of routinely measured red cell folate. In addition, it was found that serum folate is influenced by fewer analytical variables, for example C677TT polymorphism of methylenetetrahydrofolate alters the distribution of folate forms in red cells and may therefore cause analytical variability. In the evaluation of serum and red cell folate versus homocysteine, there was no evidence for the better performance of red cell folate and, of the two, serum folate appeared superior. Studies also demonstrated that very few patients would have their clinical outcome altered by the measurement of red cell folate in addition to serum folate. In relation to specific population groups, serum folate appeared to be a superior marker of folate status in vitamin B12 deficiency.(60) The authors also found that serum folate may have more sensitivity in responding to changes in folate intake (supplements or fortification) than red cell folate at both short- and long-term follow-up.(60) When blood was collected during pregnancy, serum folate appeared to predict NTD risk as well as red cell folate.(60) The authors concluded that overall, as a routine test of folate status, serum folate appears to offer the best combination of test cost and clinical information. MBS Reviews – Folate Review Report Page 32 February 2014 6 REVIEW OF THE ECONOMIC EVIDENCE FOR FOLATE TESTING This Chapter presents a preliminary economic evaluation of folate testing, which is limited to a summary of the findings from the available studies identified through the systematic literature review. A formal modelled economic evaluation of folate testing was not within the scope of this review. 6.1 Costing studies or economic analyses relevant to folate testing A simple costing analysis was undertaken in the retrospective study mentioned in Section 5.3 that analysed inpatient and emergency department serum folate tests at their institution in the United States.(62) Based on the institution’s charge for serum folate, a total of $US316,043 was charged for the 2,093 folate tests performed. The amount charged per deficient result (0.1% of tests) was $US158,022. The authors compared their results with that of a 2001 study (also a retrospective review) in patients with macrocytosis and anaemia, which reported a charge of $US1,321 per deficient test. The 100-fold difference was attributed to decreased rate of deficient tests from 2001 to 2013, due to mandatory fortification. The authors acknowledged that the actual cost to the hospital of a serum folate test was much lower due to the highly automated process, and was estimated to be <$US2,093. Because no change was made for the deficient patients in the study, the charge per serum folate deficient result that changed management approached infinity. The authors concluded that the exceptionally low utility of serum folate testing makes the costs associated with these tests excessive. MBS Reviews – Folate Review Report Page 33 February 2014 7 FINDINGS AND CONCLUSIONS This Chapter sets out the findings and conclusions of the review of folate testing – as represented by MBS item numbers 66599 and 66602 – based on the analysis of the available MBS data; evidence obtained through systematic literature review; and the information derived from the stakeholder consultations. 7.1 Current usage of folate and/or vitamin B12 testing services in Australia Over the past 10 years, the number of claims for MBS item 66599 has more than doubled (+119%) from 282,531 in 2003/04 to 618,744 in 2012/13. Over the same timeframe the number of claims for MBS item 66602 has had an even greater increase (+307%) from 522,980 to 2,129,051. The increase in benefits paid for both items reflects the increase in claims. Benefits paid for MBS item 66599 increased from $5.7m to $12.5m (+120%), whereas benefits for MBS item 66602 increased from $19.2m to $78.5m (+309%). While total benefits increased significantly, the proportion of benefits paid to each state and territory remained relatively constant over the ten-year period. The highest proportion of benefits paid over the past ten years was in New South Wales (34% of total for 66599 and 38% for 66602), followed by Victoria (30% of total for 66599 and 28% for 66602). To further explore geographical trends in testing, an analysis was conducted of the number of services per capita (i.e. per 100,000 population), according to the address at the time of claiming of the patient to whom the service was rendered. In 2012/13, there were 2,666 claims per 100,000 people enrolled in Medicare across Australia for item 66599 and 9,172 claims per 100,000 people for item 66602. South Australia had the highest rate of claiming for item 66599 per capita (3,635 claims per 100,000 population), while the lowest per capita rate was in the Northern Territory (762 claims per 100,000 population). For item 66602, the highest number of claims per capita in 2012/13 was for NSW and Victoria (over 10,000 claims per 100,000 population in both states), while Tasmania had the lowest (less than 4,000 claims per 100,000 population). MBS item numbers 66599 and 66602 are claimed by both males and females; however, females had a higher number of tests at all ages, except in the youngest age category (< 5 years). Females also had a steeper increase in testing volume than males, with the largest difference between genders in the 15-24 and 25-34 year age groups. For item 66599, the number of tests being performed in people aged 45 years and over was 76% for males and 64% for females. For item 66602, 71% of claims for males were aged 45 years and over versus 60% for females. For females, testing decreased from age 65 years. Similarly, the number of tests dropped dramatically in elderly men. For both MBS items, there was an increase in the overall number of patients tested between 2008/09 and 2012/13. However, there was very little change in the proportion of patients receiving either one test per year (92% and 90% for items 66599 and 66602, respectively), two tests per year (7.5% and 9% for items 66599 and 66602, respectively), or three or more tests per year (0.8% for item 66599 and 1.1% for item 66602). These data suggest that the majority of folate/vitamin B12 testing services are being undertaken for the purposes of screening/testing rather than monitoring. MBS Reviews – Folate Review Report Page 34 February 2014 Over the five-year time period from 2008/09 to 2012/13, there were no material changes in the pattern of requesting providers. General practitioners and other medical practitioners accounted for approximately 71% and 67% of all providers requesting item 66599 and item 66602, respectively. Approximately 14% of providers requesting folate/vitamin B12 testing were internal medicine consultant physicians. There was a large variety of other providers requesting services, but they each accounted for less than 4% of provider counts. For both items, there was an increase over the period 2008/09 to 2012/13 in the overall number of providers requesting folate/vitamin B12 testing. For item 66599, the majority of providers (97%) requested 100 or fewer tests per year. Approximately 2% of providers requested 101 to 200 tests, and the remaining providers requested more than 200 tests per year. There were a small number of providers (ranging from 36 to 62 per year) that requested more than 400 tests per year. Approximately 88% of the providers requesting item 66602 requested 100 or fewer tests per year. Approximately 7% of providers requested between 101 and 200 tests per year and 2.5% requested 201 to 300 tests per year. Between 2010/11 and 2012/13, more than 500 providers requested over 400 tests per year. 7.2 Clinical guidance on folate testing Although there are a large number of clinical practice guidelines that recommend folate supplementation, particularly in pregnant women, they do not specifically mention folate testing, which implies that testing may not be necessary in these populations. Guidelines that mention folate testing apply to particular populations. For example, NICE guidance on dementia recommends that measurement of serum folate levels are included in a basic dementia screen, performed at the time of presentation. Guidance on the diagnosis and management of chronic fatigue syndrome/myalgic encephalomyelitis from the Royal College of General Practitioners advises that folate levels should not be carried out unless a full blood count and mean cell volume show a macrocytosis. A July 2013 report from Health Quality Canada used expert consultation to identify health conditions where folate deficiency may be of concern and where folate testing may be appropriate. Based on the expert consultation, the Ontario Health Technology Advisory Committee (OHTAC) recommended that red blood cell folate testing be restricted to individuals with: low haemoglobin levels and a high mean corpuscular volume; suspected gastrointestinal disorders causing malabsorption or suspected malnutrition of any cause. 7.3 Relationship between testing for folate levels and health outcomes The main function of folate is to help form RBCs and produce DNA. Folate also prevents NTD during fetal development. A deficiency in folic acid can lead to increased homocysteine levels in plasma since folate is necessary for the conversion of homocysteine to methionine. In addition, deficiency can result in perturbation of the metabolic pathway of conversion of homocysteine to methionine with consequent disruption of DNA synthesis caused by thymidine lack resulting in megaloblastic anaemia, as well as other adverse effects on the nervous system and other organs. However, folate deficiency is much less common since the introduction of mandatory fortification with folate of wheat flour in Australia in September 2009. A retrospective study to determine the impact of the fortification program on blood MBS Reviews – Folate Review Report Page 35 February 2014 folate levels reported that the prevalence of low serum folate levels decreased from 9.3% in 2007 to 2.1% in 2010 and the prevalence of low RBC folate levels decreased from 3.4% to 0.5%. Due to the low prevalence of folate deficiency, the clinical utility of folate testing is questionable. No definitive conclusions can be drawn about the effectiveness of folate testing since no prospective trials have been conducted to directly assess the impact of testing on health outcomes in healthy populations or in patients with chronic disease associated with folate deficiency. Although retrospective studies do not meet the inclusion criteria for this review, several retrospective studies (low level evidence) were identified that examined the clinical utility of folate testing in the United States. Given the low rates of serum folate deficiency and the lack of change in management based on deficient results, the authors of the studies concluded that in folic acid fortified countries, serum folate testing has low utility for inpatients and emergency department patients or routine testing in clinical practice for patients with anaemia or dementia. 7.4 Harms associated with folate testing No trials designed to directly measure the risks or harms associated with folate testing were identified. However, folate testing relies on a blood draw, which is a safe procedure. It is likely that the consequences of inaccurate or inappropriately interpreted folate test results, such as a false positive, are relatively small. Folate supplements are generally considered safe when taken in amounts that are not higher than the recommended dietary allowance. 7.5 Quality of folate testing A poor quality systematic review sought to compare the effectiveness of serum versus red cell folate. The review did not find evidence to justify the higher cost of routinely measured red cell folate. The review found that serum folate appeared to be a superior marker of folate status in vitamin B12 deficiency and may have more sensitivity in responding to changes in folate intake (supplements or fortification) than red cell folate. Both tests appeared to predict NTD risk equally. Serum folate also has the advantage of being influenced by fewer analytical variables. In an evaluation of serum and red cell folate versus homocysteine, there was no evidence for the better performance of red cell folate and, of the two, serum folate appeared superior. Studies also demonstrated that very few patients would have their clinical outcome altered by the measurement of red cell folate in addition to serum folate. The authors concluded that overall, as a routine test of folate status, serum folate appears to offer the best combination of test cost and clinical information. 7.6 Cost implications of folate testing There was very limited and poor quality evidence relating to the cost of folate testing. In one retrospective study from the United States, the authors concluded that the exceptionally low utility of serum folate testing makes the costs associated with these tests excessive. 7.7 Conclusions There has been a substantial increase in the number of claims for folate/vitamin B12 testing over the past ten years. Analysis of MBS data indicates that the majority of vitamin B12 testing services are requested by GPs and OMPs for the purposes of screening or testing, MBS Reviews – Folate Review Report Page 36 February 2014 rather than follow-up monitoring. There are no Australian clinical practice guidelines that either advocate or recommend against routine testing for folate in any patient population. However, there is guidance from the UK recommending measurement of serum folate levels in a basic dementia screen. There is also guidance from the UK that folate levels should not be carried out in patients with chronic fatigue syndrome/myalgic encephalomyelitis unless a full blood count and mean cell volume show a macrocytosis. There are no recommendations on the frequency of folate testing and there is no prospective evidence regarding the clinical utility of folate testing in any population. However, low level evidence from retrospective studies suggests that serum folate testing has low utility in folic acid fortified countries. MBS Reviews – Folate Review Report Page 37 February 2014 APPENDIX 1 – References 1. Halsted CH. The intestinal absorption of dietary folates in health and disease. J Am Coll Nutr 1989 Dec;8(6):650-8. 2. Gregory JF, 3rd, Williamson J, Liao JF, Bailey LB, Toth JP. Kinetic model of folate metabolism in nonpregnant women consuming [2H2]folic acid: isotopic labeling of urinary folate and the catabolite para-acetamidobenzoylglutamate indicates slow, intake-dependent, turnover of folate pools. J Nutr 1998 Nov;128(11):1896-906. 3. Oh R, Brown DL. Vitamin B12 deficiency. Am Fam Physician 2003 Mar 1;67(5):979-86. 4. Green R. Indicators for assessing folate and vitamin B-12 status and for monitoring the efficacy of intervention strategies. Am J Clin Nutr 2011 Aug;94(2):666S-72S. 5. Stabler SP. Screening the older population for cobalamin (vitamin B12) deficiency. J Am Geriatr Soc 1995 Nov;43(11):1290-7. 6. Gregory Iii JF. Bioavailability of folate. Eur J Clin Nutr 1997;51(SUPPL. 1):S54-S9. 7. Pfeiffer CM, Rogers LM, Bailey LB, Gregory Iii JF. Absorption of folate from fortified cereal-grain products and of supplemental folate consumed with or without food determined by using a dual- label stable-isotope protocol. American Journal of Clinical Nutrition 1997;66(6):1388-97. 8. Australian Health and Medical Research Council and the New Zealand Ministry of Health. Nutrient reference values for Australia and New Zealand including recommended daily intakes. 2006; Available from: http://www.nrv.gov.au/nutrients/folate.htm. 9. Kaushansky K BE, Seligsohn U, Lichtman MA, Kipps TJ, Prchal JT,. Folate, cobalamin, and megaloblastic anemias. 8th ed ed. R. G, editor. New York: McGraw-Hill; 2010. 10. Halsted CH. Folate deficiency in alcoholism. Am J Clin Nutr 1980 Dec;33(12):2736-40. 11. Lindenbaum J. Folate and vitamin B12 deficiencies in alcoholism. Semin Hematol 1980 Apr;17(2):119-29. 12. Nkrumah FK, Nathoo KJ, Sanders DM. Iron, folate and vitamin B12 in severe protein-energy malnutrition. Cent Afr J Med 1988 Mar;34(3):39-43. 13. Thompson T. Folate, iron, and dietary fiber contents of the gluten-free diet. J Am Diet Assoc 2000 Nov;100(11):1389-96. 14. Malterre T. Digestive and nutritional considerations in celiac disease: could supplementation help? Altern Med Rev 2009 Sep;14(3):247-57. 15. Higgins JR, Quinlivan EP, McPartlin J, Scott JM, Weir DG, Darling MR. The relationship between increased folate catabolism and the increased requirement for folate in pregnancy. Bjog 2000 Sep;107(9):1149-54. 16. Kirke PN, Molloy AM, Daly LE, Burke H, Weir DG, Scott JM. Maternal plasma folate and vitamin B12 are independent risk factors for neural tube defects. Q J Med 1993 Nov;86(11):703-8. MBS Reviews – Folate Review Report Page 38 February 2014 17. Groenen PM, van Rooij IA, Peer PG, Gooskens RH, Zielhuis GA, Steegers-Theunissen RP. Marginal maternal vitamin B12 status increases the risk of offspring with spina bifida. Am J Obstet Gynecol 2004 Jul;191(1):11-7. 18. Suarez L, Hendricks K, Felkner M, Gunter E. Maternal serum B12 levels and risk for neural tube defects in a Texas-Mexico border population. Ann Epidemiol 2003 Feb;13(2):81-8. 19. Green R MJ. Handbook of Vitamins. 4th ed. Zempleni J RR, editor. Boca Raton, FL: Taylor & Francis Group; 2007. 20. Aarts EO, Janssen IM, Berends FJ. The gastric sleeve: losing weight as fast as micronutrients? Obes Surg 2011 Feb;21(2):207-11. 21. Schubert ML. Gastric secretion. Curr Opin Gastroenterol 2007 Nov;23(6):595-601. 22. Cravo ML, Camilo ME. Hyperhomocysteinemia in chronic alcoholism: Relations to folic acid and vitamins B6 and B12 status. Nutrition 2000;16(4):296-302. 23. Shelnutt KP, Kauwell GPA, Chapman CM, Gregory Iii JF, Maneval DR, Browdy AA, et al. Folate Status Response to Controlled Folate Intake Is Affected by the Methylenetetrahydrofolate Reductase 677C→T Polymorphism in Young Women. Journal of Nutrition 2003;133(12):4107-11. 24. Ponziani FR, Cazzato IA, Danese S, Fagiuoli S, Gionchetti P, Annicchiarico BE, et al. Folate in gastrointestinal health and disease. Eur Rev Med Pharmacol Sci 2012 Mar;16(3):376-85. 25. Yakut M, Ustun Y, Kabacam G, Soykan I. Serum vitamin B12 and folate status in patients with inflammatory bowel diseases. Eur J Intern Med 2010 Aug;21(4):320-3. 26. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet 1991 Jul 20;338(8760):131-7. 27. Lindenbaum J, Healton EB, Savage DG, Brust JC, Garrett TJ, Podell ER, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med 1988 Jun 30;318(26):1720-8. 28. Tiemeier H, van Tuijl HR, Hofman A, Meijer J, Kiliaan AJ, Breteler MM. Vitamin B12, folate, and homocysteine in depression: the Rotterdam Study. Am J Psychiatry 2002 Dec;159(12):2099-101. 29. Ramos MI, Allen LH, Mungas DM, Jagust WJ, Haan MN, Green R, et al. Low folate status is associated with impaired cognitive function and dementia in the Sacramento Area Latino Study on Aging. Am J Clin Nutr 2005 Dec;82(6):1346-52. 30. Wang HX, Wahlin A, Basun H, Fastbom J, Winblad B, Fratiglioni L. Vitamin B(12) and folate in relation to the development of Alzheimer's disease. Neurology 2001 May 8;56(9):1188-94. 31. Medicine Io, Board FaN. Dietary Reference Intakes: Thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC1998. 32. US Department of Agriculture ARS. USDA National Nutrient Database for Standard Reference, Release 24. 2011; Available from: http://www.ars.usda.gov/ba/bhnrc/ndl. 33. Food Standards ANZ. Adding vitamins and minerals to food. 2012; Available from: http://www.foodstandards.gov.au/consumerinformation/fortification.cfm. MBS Reviews – Folate Review Report Page 39 February 2014 34. K ABW. Interim evaluation of the voluntary folate fortification policy. Canberra, ACT: Australian Food and Nutrition Monitoring Unit2001. 35. 1999. Shils M OJ, Shike M, Ross AC. Folic Acid. V H, editor. Baltimore: Williams & Wilkins; 36. Standards ANZF. Standard 2.1.1 Clause 4 (2). 2009. 37. AIHW. Mandatory folic acid and iodine fortification in Australia and New Zealand. Baseline report for monitoring Canberra, ACT2011. 38. Brown RD, Langshaw MR, Uhr EJ, Gibson JN, Joshua DE. The impact of mandatory fortification of flour with folic acid on the blood folate levels of an Australian population. Med J Aust 2011 Jan 17;194(2):65-7. 39. Snow CF. Laboratory diagnosis of vitamin B12 and folate deficiency: a guide for the primary care physician. Arch Intern Med 1999 Jun 28;159(12):1289-98. 40. Cook JD, Duh S. Laboratory reference range values. 2005. 41. Guidelines on the investigation and diagnosis of cobalamin and folate deficiencies. A publication of the British Committee for Standards in Haematology. BCSH General Haematology Test Force. Clin Lab Haematol 1994 Jun;16(2):101-15. 42. NEQAS U. Haematinic assays scheme. Annual report 2001 Sutton, Coldfield2002. 43. Arsenault JE, Mora-Plazas M, Forero Y, Lopez-Arana S, Baylin A, Villamor E. Hemoglobin concentration is inversely associated with erythrocyte folate concentrations in Colombian school-age children, especially among children with low vitamin B12 status. Eur J Clin Nutr 2009 Jul;63(7):842-9. 44. Wilcken DE, Gupta VJ, Reddy SG. Accumulation of sulphur-containing amino acids including cysteine-homocysteine in patients on maintenance haemodialysis. Clin Sci (Lond) 1980 May;58(5):427-30. 45. Selhub J, Jacques PF, Wilson PW, Rush D, Rosenberg IH. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. Jama 1993 Dec 8;270(22):2693-8. 46. Jacques PF, Rosenberg IH, Rogers G, Selhub J, Bowman BA, Gunter EW, et al. Serum total homocysteine concentrations in adolescent and adult Americans: results from the third National Health and Nutrition Examination Survey. Am J Clin Nutr 1999 Mar;69(3):482-9. 47. Louey W LZ, and Sikaris KA. Which Blood Collection Tube is Better for Homocysteine. 48. Hoffbrand AV, Newcombe FA, Mollin DL. Method of assay of red cell folate activity and the value of the assay as a test for folate deficiency. J Clin Pathol 1966 Jan;19(1):17-28. 49. Galloway M, Rushworth L. Red cell or serum folate? Results from the National Pathology Alliance benchmarking review. J Clin Pathol 2003 Dec;56(12):924-6. 50. Carmel R, Green R, Rosenblatt DS, Watkins D. Update on cobalamin, folate, and homocysteine. Hematology Am Soc Hematol Educ Program 2003:62-81. MBS Reviews – Folate Review Report Page 40 February 2014 51. Tucker KL, Mahnken B, Wilson PW, Jacques P, Selhub J. Folic acid fortification of the food supply. Potential benefits and risks for the elderly population. Jama 1996 Dec 18;276(23):1879-85. 52. AGREE Next Steps Consortium. The AGREE II Instrument 2009; Available from: http://www.agreetrust.org 53. Richardson WS, Scott MD WM, et al. The well built clinical question: a key to evidence based decisions. ACP Journal Club 1995;123:ppA-12. . 54. Royal Australian College of General Practitioners. Guidelines for preventative activities in general practice. 8th edition. 2012. 55. Smellie WSA, Wilson D, McNulty CAM, Galloway MJ, Spickett GA, Finnigan DI. Best practice in primary care pathology: Review 1 J Clin Pathol 2005;58(10):1016-24. 56. National Institute for Health and Clinical Excellence. National Clinical Practice Guidelines Number 42. Dementia: A NICE-SCIE Guideline on supporting people with dementia and their carers in health and social care. 2012. 57. Turnbull N, Shaw EJ, Baker R, Dunsdon S, Costin N, Britton G, et al. Chronic fatigue syndrome/myalgic encephalomyelitis (or encephalopathy): diagnosis and management of chronic fatigue syndrome/myalgic encephalomyelitis (or encephalopathy) in adults and children. London: Royal College of General Practitioners. 2007. 58. Ontario Health Technology Advisory Committee. Health Quality Ontario. Appropriateness Phase 1 OHTAC Recommendations: Annual health exams, aspartate aminotransferase testing, ferritin testing, folate testing and vitamin B12 testing. 2013. 59. Health Quality Ontario. Folate and folic acid: an expert consultation. . 2013. 60. Farrell CJL, Kirsch SH, Herrmann M. Red cell or serum folate: What to do in clinical practice? Clinical Chemistry and Laboratory Medicine 2013;51(3):555-69. 61. Ashraf MJ, Cook JR, Rothberg MB. Clinical utility of folic acid testing for patients with anemia or dementia. Journal of General Internal Medicine 2008;23(6):824-6. 62. Theisen-Toupal J, Horowitz GL, Breu AC. Utility, charge, and cost of inpatient and emergency department serum folate testing. Journal of Hospital Medicine 2013;8(2):91-5. 63. NHMRC. NHMRC levels of evidence and grades for recommendations for developers of guidelines. [Internet]. Canberra, ACT: National Health and Medical Research Council; 2009. Available from: http://www.nhmrc.gov.au/_files_nhmrc/file/guidelines/evidence_statement_form.pdf. MBS Reviews – Folate Review Report Page 41 February 2014 APPENDIX 2 – Review Consultation Committee members As part of the MBS Review process, the Department established a Review Consultation Committee (RCC). The RCC is a time-limited committee of nominated representatives to provide advice to the Department to inform the review process, such as the development of review reports, i.e. scope and protocol documents, clinical practice and policy issues. Name A/Prof Ken Sikaris A/Prof Hans Schneider Dr Zhong Lu Dr Paul Glendenning Dr Richard Whiting Dr Andrew Boyden Dr Ie-Wen Sim Dr Peter Harman Dr Dan McLaughlin Dr Walid Jammal Chair and Secretariat MBS Reviews – Folate Review Report Representing Royal College of Pathologists of Australasia Royal College of Pathologists of Australasia Royal College of Pathologists of Australasia Royal College of Pathologists of Australasia Australian Medical Association NPS Medicinewise Endocrine Society of Australia In-vitro Diagnostic (IVD) Australia Australian and New Zealand Association of Neurologists General Practitioner MSAC Evaluation Sub-Committee (ESC) member Department of Health Page 42 February 2014 APPENDIX 3 – MBS Information The MBS item numbers for folate testing in scope of this review include 66599 and 66602 (see Table A3.1). Both of the items relate to testing serum vitamin B12 or testing red cell (or serum) folate. Both of the items are subject to Rule 21 (i.e. no more than three of any combination of these tests are eligible for Medicare subsidy per patient per year). Table A3.1: Description of folate testing funded under the MBS Item number 66599 66602 MBS item number description Serum B12 or red cell folate and, if required, serum folate (Item is subject to Rule 21) Fee: $23.60 Benefit: 75% = $17.70 85% = $20.10 Serum B12 and red cell folate and, if required, serum folate (Item is subject to Rule 21) Fee: $42.95 Benefit: 75% = $32.25 Both of the items are subject to Rule 21: 85% = $36.55 Serum B12 and red cell folate testing 21.(1) For items 66599 and 66602, a medicare benefit is not payable for more than 3 episodes of services described in item 66599 or 66602, or any combination of those items, in a 12 month period. 21.(2) A medicare benefit is not payable for a service described in item 66599 if the service was provided as part of the same patient episode as a service described in item 66602. Source: Department of Human Services – Medicare Australia, accessed September 2013 Table A3.2 shows when the MBS item numbers for folate/vitamin B12 testing were included on the MBS. Table A3.2: Item number, descriptor and schedule fee start dates for MBS item numbers MBS item number 66599 66602 Type of date Item Start Date Current Descriptor Start Date Current Schedule fee start date Item Start Date Current Descriptor Start Date Current Schedule fee start date Date 01-Nov-1998 01-Mar-1999 01-Jan-2013 01 Nov 1998 01 Mar 1999 01-Jan-2013 Source: Department of Human Services – Medicare Australia, accessed September 2013 MBS Reviews – Folate Review Report Page 43 February 2014 APPENDIX 4 – Search term strategy The literature search strategies focused on the clinical evidence for folate testing (Table A4.1) and the cost implications associated with folate testing (Table A4.2). Table A4.1: Search strategy for clinical evidence Population General healthy population Patients diagnosed with anaemia Search Terms Embase and Medline Population – ((‘pregnancy’/exp OR ‘pregnancy’) OR (‘infant’/exp OR ‘infant’) OR (‘human milk’/exp OR ‘human milk’) OR (‘lactation’/exp OR ‘lactation’) OR (‘vegetarian’/exp OR ‘vegetarian’) OR (‘malnutrition’/exp OR ‘malnutrition’) OR (‘elderly’/exp OR ‘elderly’) OR (‘aged’/exp OR ‘aged’) OR (‘gluten free diet’/exp OR ‘gluten free diet’) OR (‘alcoholism’/exp OR ‘alcoholism’)) AND Intervention – (‘folate’/exp OR ‘folate’ OR ‘folic acid’/exp OR ‘folic acid’ OR ‘vitamin B9’/exp OR ‘vitamin B9’ OR ‘tetrahydrofolic acid’ OR ‘methylenetetrahydrofolic acid’ OR ‘serum folate’/exp OR ‘serum folate’ OR’ red cell folate’/exp OR ‘red cell folate’ OR ‘erythrocyte folate’/exp OR ‘erythrocyte folate’) AND (‘testing’/exp OR ‘testing’ OR ‘haematologic test*’/exp OR ‘haematologic test*’) AND Limits – [humans]/lim AND [english]/lim Cochrane Library Population – ((MeSH descriptor Pregnancy explode all trees) OR (MeSH descriptor Infant explode all trees) OR (MeSH descriptor Human Milk explode all trees) OR (MeSH descriptor Lactation explode all trees) OR (MeSH descriptor vegetarian explode all trees) OR (MeSH descriptor Malnutrition explode all trees) OR (MeSH descriptor Aged explode all trees) OR (MeSH descriptor Alcoholism explode all trees) OR ((pregnancy) OR (pregnancy):ti,ab,kw) OR ((infant) OR (infant):ti,ab,kw) OR ((human milk) OR (human milk):ti,ab,kw) OR ((lactation) OR (lactation):ti,ab,kw) OR ((vegetarian) OR (vegetarian):ti,ab,kw) OR ((malnutrition) OR (malnutrition):ti,ab,kw) OR ((elderly) OR (eldrely):ti,ab,kw) OR ((aged) OR (aged):ti,ab,kw) OR ((gluten free diet) OR (gluten free diet):ti,ab,kw) OR ((alcoholism) OR (alcoholism):ti,ab,kw)) AND Intervention – (MeSH descriptor Folate explode all trees) OR (folate):ti,ab,kw OR (MeSH descriptor Folic acid explode all trees) OR (folic acid):ti,ab,kw) ) OR (MeSH descriptor Vitamin B9 explode all trees) OR (vitamin B9):ti,ab,kw OR (MeSH descriptor Tetrahydrofolic acid explode all trees) OR (tetrahydrofolic acid):ti,ab,kw) ) OR (MeSH descriptor Methylenetetrahydrofolic acid explode all trees) OR (methylenetetrahydrofolic acid):ti,ab,kw OR (MeSH descriptor Serum folate explode all trees) OR (serum folate):ti,ab,kw) ) OR (MeSH descriptor Red cell folate explode all trees) OR (red cell folate):ti,ab,kw OR (MeSH descriptor Erythrocyte folate explode all trees) OR (erythrocyte folate):ti,ab,kw) ) AND ((MeSH descriptor Testing explode all trees) OR (Testing):ti,ab,kw OR (MeSH descriptor Haematologic test* explode al trees) OR (Haematologic test*):ti,ab,kw) AND Limits [humans]/lim AND [english]/lim Embase and Medline Population – ((‘anaemia’/exp OR ‘anaemia’ OR ‘anemia’/exp OR ‘anemia’) OR (‘macrocyt*’/exp OR ‘macrocyt*)’ OR (‘megaloblastic ’/exp OR ‘megaloblastic’) OR (‘pernicious’/exp OR ‘pernicious’) OR (‘pancytopenia’/exp OR ‘pancytopenia’)) AND NOT (‘iron deficiency anaemia’/exp OR ‘iron deficiency anaemia’) AND Intervention – (‘folate’/exp OR ‘folate’ OR ‘folic acid’/exp OR ‘folic acid’ OR MBS Reviews – Folate Review Report Page 44 February 2014 Population Patients with neurologic disease Search Terms ‘vitamin B9’/exp OR ‘vitamin B9’ OR ‘tetrahydrofolic acid’ OR ‘methylenetetrahydrofolic acid’ OR ‘serum folate’/exp OR ‘serum folate’ OR’ red cell folate’/exp OR ‘red cell folate’ OR ‘erythrocyte folate’/exp OR ‘erythrocyte folate’) AND (‘testing’/exp OR ‘testing’ OR ‘haematologic test*’/exp OR ‘haematologic test*’) AND Limits – [humans]/lim AND [english]/lim Cochrane Library Population – ((MeSH descriptor Anaemia explode all trees) OR (MeSH descriptor Megaloblastic explode all trees) OR (MeSH descriptor Pernicious explode all trees) OR (MeSH descriptor Pancytopenia explode all trees) OR ((anaemia) OR (anaemia):ti,ab,kw) OR ((megaloblastic) OR (megaloblastic):ti,ab,kw) OR (macrocyt*) OR ((pernicious) OR (pernicious):ti,ab,kw) OR ((pancytopenia) OR (pancytopenia):ti,ab,kw) ) AND NOT ((MeSH descriptor Iron deficiency anaemia) OR (iron deficiency anaemia):ti,ab,kw) AND Intervention –((MeSH descriptor Folate explode all trees) OR (folate):ti,ab,kw OR (MeSH descriptor Folic acid explode all trees) OR (folic acid):ti,ab,kw) ) OR (MeSH descriptor Vitamin B9 explode all trees) OR (vitamin B9):ti,ab,kw OR (MeSH descriptor Tetrahydrofolic acid explode all trees) OR (tetrahydrofolic acid):ti,ab,kw) ) OR (MeSH descriptor Methylenetetrahydrofolic acid explode all trees) OR (methylenetetrahydrofolic acid):ti,ab,kw OR (MeSH descriptor Serum folate explode all trees) OR (serum folate):ti,ab,kw) ) OR (MeSH descriptor Red cell folate explode all trees) OR (red cell folate):ti,ab,kw OR (MeSH descriptor Erythrocyte folate explode all trees) OR (erythrocyte folate):ti,ab,kw) ) AND ((MeSH descriptor Testing explode all trees) OR (Testing):ti,ab,kw OR (MeSH descriptor Haematologic test* explode al trees) OR (Haematologic test*):ti,ab,kw) AND Limits [humans]/lim AND [english]/lim Embase and Medline Population – ((‘paresthesias’/exp OR ‘paresthesias’) OR (‘peripheral neuropathy’/exp OR ‘peripheral neuropathy’) OR (‘combined system disease’/exp OR ‘combined systems disease’)) AND Intervention – ‘folate’/exp OR ‘folate’ OR ‘folic acid’/exp OR ‘folic acid’ OR ‘vitamin B9’/exp OR ‘vitamin B9’ OR ‘tetrahydrofolic acid’ OR ‘methylenetetrahydrofolic acid’ OR ‘serum folate’/exp OR ‘serum folate’ OR’ red cell folate’/exp OR ‘red cell folate’ OR ‘erythrocyte folate’/exp OR ‘erythrocyte folate’) AND (‘testing’/exp OR ‘testing’ OR ‘haematologic test*’/exp OR ‘haematologic test*’) AND Limits – [humans]/lim AND [english]/lim Cochrane Library Population – ((MeSH descriptor Paresthesias explode all trees) OR (MeSH descriptor Peripheral Neuropathy explode all trees) OR (MeSH descriptor Combined Systems Disease explode all trees) OR ((paresthesias) OR (paresthesias):ti,ab,kw) OR ((peripheral neuropathy) OR (peripheral neuropathy):ti,ab,kw) OR ((combined systems disease) OR (combined systems disease):ti,ab,kw)) AND Intervention – (MeSH descriptor Folate explode all trees) OR (folate):ti,ab,kw OR (MeSH descriptor Folic acid explode all trees) OR (folic acid):ti,ab,kw) ) OR (MeSH descriptor Vitamin B9 explode all trees) OR (vitamin B9):ti,ab,kw OR (MeSH descriptor Tetrahydrofolic acid explode all trees) OR (tetrahydrofolic acid):ti,ab,kw) ) OR (MeSH descriptor Methylenetetrahydrofolic acid explode all trees) OR (methylenetetrahydrofolic acid):ti,ab,kw OR (MeSH descriptor Serum folate explode all trees) OR (serum folate):ti,ab,kw) ) OR (MeSH descriptor Red MBS Reviews – Folate Review Report Page 45 February 2014 Population Patients with gastrointestinal and malabsoption diseases Search Terms cell folate explode all trees) OR (red cell folate):ti,ab,kw OR (MeSH descriptor Erythrocyte folate explode all trees) OR (erythrocyte folate):ti,ab,kw) ) AND ((MeSH descriptor Testing explode all trees) OR (Testing):ti,ab,kw OR (MeSH descriptor Haematologic test* explode al trees) OR (Haematologic test*):ti,ab,kw) AND Limits [humans]/lim AND [english]/lim Embase and Medline Population – ((‘atrophic body gastritis’/exp OR ‘atrophic body gastritis’) OR (‘gastrectomy’/exp OR ‘gastrectomy’) OR (‘gastric sleeve’/exp OR ‘gastric sleeve’) OR (‘peptic ulcer’/exp OR ‘peptic ulcer’) OR (‘H. Pylori’/exp OR ‘H. Pylori’) OR (‘dyspepsia’/exp OR ‘dyspepsia’) OR (‘diarrhoea’/exp OR ‘diarrhoea’) OR (‘coeliac disease’/exp OR ‘coeliac disease’) OR (‘Crohn’s disease’/exp OR ‘Crohn’s disease’) OR (‘tapeworms’/exp OR ‘tapeworms’)) AND Intervention – (‘folate’/exp OR ‘folate’ OR ‘folic acid’/exp OR ‘folic acid’ OR ‘vitamin B9’/exp OR ‘vitamin B9’ OR ‘tetrahydrofolic acid’ OR ‘methylenetetrahydrofolic acid’ OR ‘serum folate’/exp OR ‘serum folate’ OR’ red cell folate’/exp OR ‘red cell folate’ OR ‘erythrocyte folate’/exp OR ‘erythrocyte folate’) AND (‘testing’/exp OR ‘testing’ OR ‘haematologic test*’/exp OR ‘haematologic test*’) AND Limits – [humans]/lim AND [english]/lim Cochrane Library Population – ((MeSH descriptor Atrophic Body Gastritis explode all trees) OR (MeSH descriptor Gastrectomy explode all trees) OR (MeSH descriptor Gastric Sleeve explode all trees) OR (MeSH descriptor Peptic Ulcer explode all trees) OR (MeSH descriptor H. pylori explode all trees) OR (MeSH descriptor Dyspepsia explode all trees) OR (MeSH descriptor Diarrhoea explode all trees) OR (MeSH descriptor Coeliac Disease explode all trees) OR (MeSH descriptor Crohn’s Disease explode all trees) OR (MeSH descriptor Tapeworms explode all trees) OR ((atrophic body gastritis) OR (atrophic body gastritis):ti,ab,kw OR (gastrectomy) OR (gastrectomy):ti,ab,kw OR (gastric sleeve) OR (gastric sleeve):ti,ab,kw OR (peptic ulcer) OR (peptic ulcer):ti,ab,kw OR (h. pylori) OR (h. pylori):ti,ab,kw OR (dyspepsia) OR (dyspepsia):ti,ab,kw OR (diarrhoea) OR (diarrhoea):ti,ab,kw OR (coeliac disease) OR (coeliac disease):ti,ab,kw OR (Crohn’s disease) OR (Crohn’s disease):ti,ab,kw OR (tapeworms) OR (tapeworms):ti,ab,kw ) AND Intervention – ((MeSH descriptor Folate explode all trees) OR (folate):ti,ab,kw OR (MeSH descriptor Folic acid explode all trees) OR (folic acid):ti,ab,kw) ) OR (MeSH descriptor Vitamin B9 explode all trees) OR (vitamin B9):ti,ab,kw OR (MeSH descriptor Tetrahydrofolic acid explode all trees) OR (tetrahydrofolic acid):ti,ab,kw) ) OR (MeSH descriptor Methylenetetrahydrofolic acid explode all trees) OR (methylenetetrahydrofolic acid):ti,ab,kw OR (MeSH descriptor Serum folate explode all trees) OR (serum folate):ti,ab,kw) ) OR (MeSH descriptor Red cell folate explode all trees) OR (red cell folate):ti,ab,kw OR (MeSH descriptor Erythrocyte folate explode all trees) OR (erythrocyte folate):ti,ab,kw) ) AND ((MeSH descriptor Testing explode all trees) OR (Testing):ti,ab,kw OR (MeSH descriptor Haematologic test* explode al trees) OR (Haematologic test*):ti,ab,kw) AND Limits [humans]/lim AND [english]/lim MBS Reviews – Folate Review Report Page 46 February 2014 Population Patients with psychiatric disorders Search Terms Embase and Medline Population – ((‘dementia’/exp OR ‘dementia’) OR (‘depression’/exp OR ‘depression’) OR (‘psychosis’/exp OR ‘psychosis’) OR (‘Alzheimer’s disease’/exp OR ‘Alzheimer’s disease’)) AND Intervention – ‘folate’/exp OR ‘folate’ OR ‘folic acid’/exp OR ‘folic acid’ OR ‘vitamin B9’/exp OR ‘vitamin B9’ OR ‘tetrahydrofolic acid’ OR ‘methylenetetrahydrofolic acid’ OR ‘serum folate’/exp OR ‘serum folate’ OR’ red cell folate’/exp OR ‘red cell folate’ OR ‘erythrocyte folate’/exp OR ‘erythrocyte folate’) AND (‘testing’/exp OR ‘testing’ OR ‘haematologic test*’/exp OR ‘haematologic test*’) AND Limits – [humans]/lim AND [english]/lim Cochrane Library Population – ((MeSH descriptor Dementia explode all trees) OR (MeSH descriptor Depression explode all trees) OR (MeSH descriptor Psychosis explode all trees) OR (MeSH descriptor Alzheimer’s disease explode all trees) OR((dementia) OR (dementia):ti,ab,kw) OR ((depression) OR (depression):ti,ab,kw) OR ((psychosis) OR (psychosis):ti,ab,kw) OR ((Alzheimer’s disease) OR (Alzheimer’s disease):ti,ab,kw)) AND Intervention –((MeSH descriptor Folate explode all trees) OR (folate):ti,ab,kw OR (MeSH descriptor Folic acid explode all trees) OR (folic acid):ti,ab,kw) ) OR (MeSH descriptor Vitamin B9 explode all trees) OR (vitamin B9):ti,ab,kw OR (MeSH descriptor Tetrahydrofolic acid explode all trees) OR (tetrahydrofolic acid):ti,ab,kw) ) OR (MeSH descriptor Methylenetetrahydrofolic acid explode all trees) OR (methylenetetrahydrofolic acid):ti,ab,kw OR (MeSH descriptor Serum folate explode all trees) OR (serum folate):ti,ab,kw) ) OR (MeSH descriptor Red cell folate explode all trees) OR (red cell folate):ti,ab,kw OR (MeSH descriptor Erythrocyte folate explode all trees) OR (erythrocyte folate):ti,ab,kw) ) AND ((MeSH descriptor Testing explode all trees) OR (Testing):ti,ab,kw OR (MeSH descriptor Haematologic test* explode al trees) OR (Haematologic test*):ti,ab,kw) AND Limits [humans]/lim AND [english]/lim Table A4.2: Search strategy for economic evidence Population Search Terms Patients undertaking folate testing Embase and Medline Intervention – (‘folate’/exp OR ‘folate’ OR ‘folic acid’/exp OR ‘folic acid’ OR ‘vitamin B9’/exp OR ‘vitamin B9’ OR ‘tetrahydrofolic acid’ OR ‘methylenetetrahydrofolic acid’ OR ‘serum folate’/exp OR ‘serum folate’ OR’ red cell folate’/exp OR ‘red cell folate’ OR ‘erythrocyte folate’/exp OR ‘erythrocyte folate’) AND (‘testing’/exp OR ‘testing’ OR ‘haematologic test*’/exp OR ‘haematologic test*’) AND Economic Terms – (‘economic aspect’/exp OR ‘cost benefit analysis’ OR cost* OR ‘cost effectiveness’) AND Limits – [humans]/lim AND [english]/lim Cochrane Library Intervention – (MeSH descriptor Folate explode all trees) OR (folate):ti,ab,kw OR (MeSH descriptor Folic acid explode all trees) OR (folic acid):ti,ab,kw) ) OR (MeSH descriptor Vitamin B9 explode all trees) OR (vitamin B9):ti,ab,kw OR (MeSH descriptor Tetrahydrofolic acid explode all trees) OR (tetrahydrofolic acid):ti,ab,kw) ) OR (MeSH descriptor Methylenetetrahydrofolic acid explode all trees) OR (methylenetetrahydrofolic acid):ti,ab,kw OR (MeSH descriptor Serum MBS Reviews – Folate Review Report Page 47 February 2014 Population Search Terms folate explode all trees) OR (serum folate):ti,ab,kw) ) OR (MeSH descriptor Red cell folate explode all trees) OR (red cell folate):ti,ab,kw OR (MeSH descriptor Erythrocyte folate explode all trees) OR (erythrocyte folate):ti,ab,kw) ) AND ((MeSH descriptor Testing explode all trees) OR (Testing):ti,ab,kw OR (MeSH descriptor Haematologic test* explode al trees) OR (Haematologic test*):ti,ab,kw) AND Economic Terms – (((economic aspect) OR (economic aspect):kw) OR ((cost benefit) OR (cost benefit):kw)) OR ((cost effectiveness) OR (cost effectiveness):kw) OR (MeSH descriptor Cost-Benefit Analysis explode all trees) OR (MeSH descriptor Costs and Cost Analysis explode all trees)) AND Limits [humans]/lim AND [english]/lim MBS Reviews – Folate Review Report Page 48 February 2014 APPENDIX 5 – Tools for assessing the evidence in the systematic review Table A5.1: NHMRC Dimensions of Evidence(63) Type of evidence Strength of the evidence Level Quality Statistical precision Definition The study design used, as an indicator of the degree to which bias has been eliminated by design. The methods used by investigators to minimise bias within a study design. The p-value or, alternatively, the precision of the estimate of the effect (as indicated by the confidence interval). It reflects the degree of certainty about the existence of a true effect. Size of effect The distance of the study estimate from the “null” value and the inclusion of only clinically important effects in the confidence interval. Relevance of evidence The usefulness of the evidence in clinical practice, particularly the appropriateness of the outcome measures used. Table A5.2: NHMRC designations of levels of evidence for an intervention (NHMRC)(63) Level I Intervention A systematic review of level II studies II A randomised controlled trial III-1 A pseudo randomised controlled trial (i.e. alternate allocation or some other method) III-2 A comparative study with concurrent controls: Non-randomised, experimental trial Cohort study Case-control study Interrupted time series with a control group A comparative study without concurrent controls: Historical control study Two or more single arm study Interrupted time series without a parallel control group Case series with either post-test or pre-test/post-test outcomes III-3 IV Source: Hierarchies adapted and modified from: NHMRC 1999; Bandolier 1999; Lijmer et al. 1999; Phillips et al. 2001 MBS Reviews – Folate Review Report Page 49 February 2014 Table A5.3: NHMRC quality criteria for RCTs, cohort studies, case-control studies and systemic reviews(63) Study type Randomised controlled trialsa Cohort studiesb Case-control studiesb Systematic reviewsc Quality criteria Was the study double blinded? Was allocation to treatment groups concealed from those responsible for recruiting the subjects? Were all randomised participants included in the analysis? How were subjects selected for the ‘new intervention’? How were subjects selected for the comparison or control group? Does the study adequately control for demographic characteristics, clinical features and other potential confounding variables in the design or analysis? Was the measurement of outcomes unbiased (i.e. blinded to treatment group and comparable across groups)? Was follow-up long enough for outcomes to occur? Was follow-up complete and were there exclusions from the analysis? How were cases defined and selected? How were controls defined and selected? Does the study adequately control for demographic characteristics and important potential confounders in the design or analysis? Was measurement of exposure to the factor of interest (e.g. the new intervention) adequate and kept blinded to case/control status? Were all selected subjects included in the analysis? Was an adequate search strategy used? Were the inclusion criteria appropriate and applied in an unbiased way? Was a quality assessment of included studies undertaken? Were the characteristics and results of the individual studies appropriately summarised? Were the methods for pooling the data appropriate? Were sources of heterogeneity explored? Source: National Health and Medical Research Council (NHMRC), 2000. How to review the evidence: systematic identification and review of the scientific literature, NHMRC, Commonwealth of Australia, Canberra. a Based on work of Schulz et al (1995) and Jadad et al (1996) b Based on quality assessment instruments developed and being tested in Australia and Canada c Based on articles by Greenhalgh (1997) and Hunt and McKibbon (1997). MBS Reviews – Folate Review Report Page 50