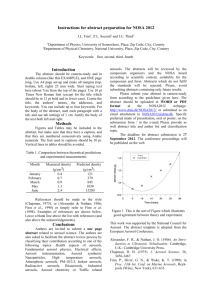
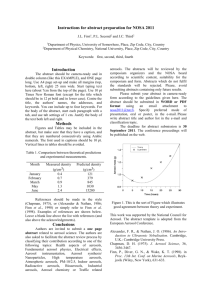
Synthesis, isolation and identification of beta
advertisement

Oxidation products of terpenes: synthesis of reference compounds and novel sampling technique Jevgeni Parshintsev Joonas Nurmi, Kari Hartonen and Marja-Liisa Riekkola University of Helsinki Finland Background • Aerosols are relatively stable suspension of solid or liquid particle in a gas, diameter ~0.002µm – 100 µm • Either directly emitted into the atmosphere or formed there by chemical reactions (primary and secondary aerosols) Impact on the energy balance of the planet direct -scattering & -absorption of solar radiation In the atmosphere indirect alter the: -formation -precipitation efficiency & -optical properties of clouds Aerosols are cooling the Earth’s surface immediately below them • Directly: I e bext l I0 Where I and I0 are the incident and transmitted light intensities respectively, L is the pathlength of the light beam, and bext is the extinction coefficient, (length)-1 bext bg b p bext bag bsg bap bsp Light extinctions due to gaseous and particulate absorption and scattering BUT: light absorption by NO2(one,which contribute significantly) is usually much Less than the total light scattering and absorption by particles Scattering is significant if D≈λ -and indirectly: Aerosol particles are “seeds” to start the formation of cloud droplets. Aerosol concentration increase => the water gets spread over many more particles => smaller drops Clouds with smaller drop: -reflect more sunlight -such clouds last longer Both effects increase the amount of sunlight that is reflected to space without reaching the surface. It is thought that aerosol cooling may partially offset expected global warming that is attributed to increases in the amount of carbon dioxide from human activity… Atmospheric aerosols http://www.ems.psu.edu/~lno/Meteo437/Aerosol.jpg, 31.7.06 Why sesquiterpenes? • ~9% of all non-methan VOCs in aerosols • almost unknown (compare to monoterpenes) • Have much higher aerosol potential than monoterpenes. Example: • 2% of total biogenic VOC- emission is assumed to be β-caryophyllene in Scandinavian atmosphere, the contribution by caryophyllene to the total biogenic secondary aerosol formation was estimated to be 12%! C15H24 Analysis of sesquiterpenes oxidation products • Usually collected on impactor plate or filter • Extracted by proper solvent • Analyzed by GC-MS (derivatization is needed if too polar) • Identification & quantitation are almost impossible nowadays because of: • Leak of reference materials, small amounts & great variety Conventional collection techniques • Impactor, filter (usually quartz) http://jolisfukyu.tokai-sc.jaea.go.jp/fukyu/tayu/ACT03E/img/09IMG/09_01.jpg http://www.menlh.go.id/apec_vc/osaka/eastjava/hap_en/filter/5-3.gif 18.11.07 Synthesis, isolation • Solution of β-caryophyllene (~1000ppm) in dichloromethane was ozonolyzed by bubbling the ozone (produced by corona discharge) through the mixture • Reaction was stopped when potassium iodide solution trap turned yellow (unreacted ozone, reaction time appr. 2h) O3 2KI H2O I2 2KOH O2 • 100 mg of Zn-powder and 10 ml of acetic acid/water mixture was added and refluxed for 1h • Compounds were extracted by LLE to the dichloromethane • Products were isolated and purified by flashchromatography Scheme of oxidation of β-caryophyllene Paineistettu pylväskromatografialaitteisto Identification • Mass-spectrometry with ESI- and EIionization and nuclear magnetic resonance spectroscopy were applied Structures O H3C O 10 11 H3C O 12 11 H2C 4 9 10 3 8 9 H H3C 7 H3C 6 3 4 5 H 5 2 H 1 O 5 H3C 8 H3C 7 6 H 6 Picture 1. Structures of β-nocaryophyllone aldehyde (left) and β-caryophyllene aldehyde (right) confirmed by NMR. 2 1 O Analysis of aerosol samples • Self made standards were used in the analysis of ambient aerosol samples collected in Hyytiälä in 2003 • GC-MS and GC*GC-FID were used • β-nocaryophyllone aldehyde was positively identified in one sample with amount of 17ng/m3 Parshintsev et al. accepted to Analytical and Bioanalytical Chemistry Conclusions I • Two β-caryophyllene oxidation products were synthesized, isolated and analyzed for the precise structures for the first time • Standards were used in analysis of ambient aerosol samples • Presence of studied compounds in the samples proves their participation in biogenic aerosol formation • However, more oxidation products of terpenes should be synthesized, to make their analysis possible, in order to understand the contribution of sesquiterpenes oxidation products to the biogenic aerosol chemistry Part II. Working principles of PILS • particle growth in a mixing condensation particle counter • droplet collection by a single jet inertial impactor • used off-line, but continuous sample flow can provide on-line applications through SPE to HPLC or directly to API-MS PILS Weber et al. Aerosol Science and Technology 35: 718–727 (2001) PILS • Sample air flow 16.7 L/min (can be increased) • Transport flow can be changed to relatively unpolar solvent • Size separation before PILS, such as virtual impactor • Gases removed by KOH, H3PO4, XADdenuders Disadvantages • Needs to be cleaned frequently (can be automated by running methanol/water steam through the system) • 10% of gases passes through the denuders => “zero” samples should be run often Aerosol sampling • Samples were collected during spring and summer 2007 in Hyytiälä (SMEARIIstation), Finland • Filter sampling was used as reference • Samples were analyzed off-line: LLE to dichloromethane, concentration, GC-MS Parshintsev et al. (2007) Manuscript in preparation Preliminary results • Pinonaldehyde was found in all samples in great amounts • Correlation with even/non-event was noticed due to the better time resolution • Quantitation of other compounds is in progress Comparison with filter sampling • Two hours filter samples showed amounts under LOD • Only day or night samples contained amounts exceeding LOD Event week (except 103) 70,00 60,00 50,00 40,00 30,00 20,00 10,00 0,00 102,00 103,00 104,00 105,00 106,00 107,00 108,00 109,00 Non-event week 70,00 60,00 50,00 40,00 30,00 20,00 10,00 0,00 113,00 114,00 115,00 116,00 117,00 118,00 119,00 Conclusions II • Time resolution of PILS is better than of filter sampling • Less artifacts • On-line possibility • Transport flow can be 2-propanol (improves the collection efficiency for less polar compounds) • However, optimization is still needed