CENG151 Introduction to Materials Science and Selection Tutorial 7
advertisement
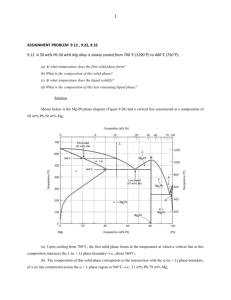
CENG151 Introduction to Materials Science and Selection Tutorial 7 16th November, 2007 Reviewing Phase Diagram Terms Phase - Any portion including the whole of a system, which is physically homogeneous within it and bounded by a surface so that it is mechanically separable from any other portions. Component – The distinct chemical substance from which the phase is formed. Phase Diagram - A diagram (map) showing phases present under equilibrium conditions and the phase compositions at each combination of temperature and overall composition. Gibbs Phase Rule - Used to determine the degrees of freedom (F), the number of independent variables available to the system: F = C – P + 1 (for pressure at constant 1 atm.) Solubility terms Solubility - The amount of one material that will completely dissolve in a second material without creating a second phase. Unlimited solubility - When the amount of one material that will dissolve in a second material without creating a second phase is unlimited. Limited solubility - When only a maximum amount of a solute material can be dissolved in a solvent material. In-Class Exercise: Revision Apply the Gibbs phase rule to a sketch of the MgO-Al2O3 phase diagram. (Figure 9-26) Find out for each phase compartment the degrees of freedom. Solution 9.5 F=2-1+1=2 F=1-2+1=0 (F=2-2+1=1) F=2-1+1=2 F=2-1+1=2 F=2-2+1=1 F=2-2+1=1 F=1-2+1=0 Phase diagram for unlimited solubility The composition of the phases in a two-phase region of the phase diagram are determined by a tie line (horizontal line connecting the phase compositions) at the system temperature. Eutectic Diagram with No Solid Solution Another binary system with components that are so dissimilar that their solubility in each other in nearly negligible. There exists a 2-phase region for pure solids (A+B). Because A and B cannot dissolve in each other! The eutectic temperature (eutektos greek for “easily melted”) is the temperature that eutectic composition is fully melted. Eutectic diagram with limited solid solution Many binary systems are partially soluble intermediate phase diagram of the previous two! α and individual phases are single solid solution phases! While α+ is a 2-phase region for pure solids α and . Eutectic Diagram with Limited Solid Solution Lead (Pb) – Tin (Sn) equilibrium phase diagram. (c)2003 Brooks/Cole, a division of Thomson Learning, Inc. Thomson Learning ™ is a trademark used herein under license. Solidification and microstructure of a Pb-2% Sn alloy. The alloy is a single-phase solid solution. (c)2003 Brooks/Cole, a division of Thomson Learning, Inc. Thomson Learning ™ is a trademark used herein under license. Solidification, precipitation, and microstructure of a Pb-10% Sn alloy. Some dispersion strengthening occurs as the β solid precipitates. The Lever Rule Tells us the amount of each phase there are in the alloy wt% C0 must be made up of appropriate amounts of α at composition Cα and of liquid at composition CLiq. The Lever Rule Basically, the proportion of the phases present are given by the relative lengths of the tie line. So, the proportions of α and L present on the diagram are: x x m m m x x m m m x x x x 9.9 9.10 So, the left side of the tie line gives the proportion of the liquid phase, and the right side of the tie line gives the proportion of the alpha phase! Example: Application of Lever Rule Calculate the amounts of and L at 1250oC in the Cu-40% Ni alloy shown in the figure below. A tie line 1250°C in the coppernickel system that is used to find the amount of each phase. ©2003 Brooks/Cole, a division of Thomson Learning, Inc. Thomson Learning™ is a trademark used herein under license. Example: Solution x = mass fraction of the alloy that is in α phase. Since we have only two phases, α and L. Thus, the mass fraction of nickel in liquid will be 1 - x. Total mass of nickel in 100 grams of the alloy = mass of nickel in liquid + mass of nickel in α So, 100 (% Ni in alloy = [(100)(1 – x)](% Ni in L) + (100)[x](% Ni in α ) x = (40-32)/(45-32) = 8/13 = 0.62 If we convert from mass fraction to mass percent, the alloy at 1250oC contains 62% α and 38% L. Note that the concentration of Ni in α phase (at 1250oC) is 45% and concentration of nickel in liquid phase (at 1250oC) is 32% (read from the diagram). Example: Limited Solubility Consider a 50:50 Pb – Sn solder. (a)For a temperature of 200˚C, determine (i) the phases present, (ii) their compositions, and (iii) their relative amounts (expressed in weight percent). (b)Repeat part (a) for 100˚C. Example: Solution (a)Reading off the phase diagram at 50wt% and 200˚C, (i) Phases present are and liquid. (ii)The composition of is ~18wt% Sn and of L is ~ 54wt% Sn. (iii)Using the Eqn 9.9 and 9.10, we have: Lever Rule x x m m m x x 9.9 x x m m x x 9.10 m xL x 54 50 wt % 100% 100% xL x 54 18 11.1% wt % L x x 50 18 100% 100% xL x 54 18 88.9% Example: Solution (cont.) (b) Similarly, at 100C, we obtain: (i) Phases are and . (ii) is ~5wt% Sn and is ~ 99wt% Sn. (iii) x x 99 50 wt % 100% 100% 52.1% x x 99 5 x x 50 5 wt % 100% 100% 47.9% x x 99 5 ©2003 Brooks/Cole, a division of Thomson Learning, Inc. Thomson Learning ™ is a trademark used herein under license. The change in structure of a Cu-40% Ni alloy during nonequilibrium solidification. Insufficient time for diffusion in the solid produces a segregated structure. In-Class Exercise: Limited Solubility Consider 1kg of an aluminum casting alloy with 10wt% silicon. (a)Upon cooling, at what temperature would the first solid appear? (b)What is the first solid phase and what is its composition? (c) At what temperature will the alloy completely solidify? (d)How much proeutectic phase will be found in the microstructure? (e)How is the silicon distributed in the microstructure at 576C? In-Class Exercise: Solution Follow microstructural development with the aid of the phase diagram: (a)For this composition, the liquidus is at ~595C. (b) It is solid solution with the composition of ~1wt% Si. (c) At the eutectic temperature, 577C. (d) Practically all of the proeutectic will have developed by 578C. Using the equation: (eqn9.9), we obtain: xL x 12.6 10 1kg 1kg m xL x 12.6 1.6 0.236kg 236 g In-Class Exercise: Solution (cont.) (e) At 576C, the overall microstructure is + . The amounts of each are: x x 1kg 100 10 1kg m x x 100 1.6 0.915kg 915 g m x x 1kg 10 1.6 1kg x x 100 1.6 0.085kg 85 g But we found in (d) that 236g of the is in the form of relatively large grains of proeutectic phase, giving: eutectic total proeutectic 915 g 236 g 679 g In-Class Exercise: Solution (cont.) The silicon distribution is then given by multiplying its weight fraction in each microstructural region by the amount of that region: Si in proeutectic = (0.016)(236g) = 3.8g Si in eutectic = (0.016)(679g) = 10.9g Si in eutectic = (1.000)(85g) = 85.0g Finally, note that the total mass of silicon in the three regions sums to 99.7g rather than 100.0g (=10wt% of the total alloy) due to round-off errors. Question 9.10 (c) (b) (a) Describe qualitatively the microstructural development during the slow cooling of a melt composed of the following compositions. (See Figure 9-16) (a)10 wt% Pb – 90 wt% Sn, (b)40 wt% Pb – 60 wt% Sn, (c)50 wt% Pb – 50 wt% Sn. (c)2003 Brooks/Cole, a division of Thomson Learning, Inc. Thomson Learning ™ is a trademark used herein under license. Solution 9.10 (a) The first solid to precipitate is solid solution, -Sn near 210˚C. At the eutectic temperature (183˚C), the remaining liquid solidifies leaving a two phase microstructure microstructure of solid solutions α-Pb and -Sn. (b) The first solid to precipitate is solid solution α-Pb near 188˚C. At the eutectic temperature (183˚C), the remaining liquid solidifies leaving a two phase microstructure of solid solution α-Pb and -Sn. (c) The first solid to precipitate is solid solution α-Pb near 210˚C. At the eutectic temperature (183˚C), the remaining liquid solidifies leaving a two-phase microstructure of solid solution α-Pb and -Sn. Question 9.13 Describe qualitatively the microstructural development that will occur upon the slow cooling of a melt composed of the following compositions. (See Figure 9-28) (a) 20 wt% Mg – 80 wt% Al, (b) 80 wt% Mg – 20 wt% Al. (a) (b) Solution 9.13 (a) The first solid to precipitate is α. At eutectic temperature (450˚C), the remaining liquid solidifies leaving a two-phase microstructure of solid solutions α and β. (b) The first solid to precipitate is . At the eutectic temperature (437˚C), the remaining liquid solidifies leaving a two-phase microstructure of solid solution and . Question 9.17 Calculate the amount of each phase present in 1kg of a 50 wt% Ni – 50 wt% Cu alloy at the following temperatures. (See Figure 9-9) (a)1400˚C (b)1300˚C (c)1200˚C Solution 9.17 (a) In the single (L) region: (c) In the single (α) mL 1kg, m 0kg (b) Two phases exist: x x 58 50 1kg 1kg mL x xL 58 46 0.667 kg 667 g x xL 50 46 1kg 1kg m x xL 58 46 0.333kg 333 g phase region: mL 0kg, m 1kg Question 9.18 Calculate the amount of each phase present in 1 kg of a 50 wt% Pb – 50 wt% Sn solder alloy at the following temperatures. (See Figure 9-16) (a)300˚C (b)200˚C (c)100˚C (d)0˚C (c)2003 Brooks/Cole, a division of Thomson Learning, Inc. Thomson Learning ™ is a trademark used herein under license. Solution 9.18 (a) In the single (L) region: mL 1kg, m 0kg (b) Two phases exist (α-Pb + L): x x Pb 50 18 1kg 1kg mL xL x Pb 54 18 0.889kg 889 g m Pb xL x 54 50 1kg 1kg xL x Pb 54 18 0.111kg 111g Solution 9.18 (cont.) (c) Two phases exist (α-Pb + -Sn): m Pb x Sn x 99 50 1kg 1kg x Sn x Pb 99 5 0.521kg 521g x x Pb 50 5 1kg 1kg m Sn x Sn x Pb 99 5 0.479kg 479 g m Pb (d) Two phases exist (α-Pb + α-Sn): x Sn x 100 50 1kg 1kg x Sn x Pb 100 1 0.505kg 505 g m Sn x x Pb 50 1 1kg 1kg x Sn x Pb 100 1 0.495kg 495 g End of Tutorial 7 Thank You!