Percent Composition (Section 11.4)
advertisement

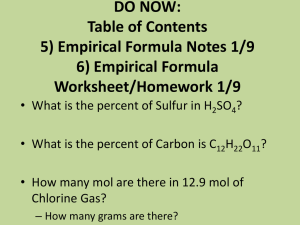
Percent Composition (Section 11.4) • Helps determine identity of unknown compound – Think CSI—they use a mass spectrometer • Percent by mass of each element in a compound mass of element percent by mass x 100 mass of compound To calculate percent composition • H2O percent by mass mass of element x 100 mass of compound Percent Composition of Water • Even though there are 2 hydrogen atoms in water, most of the mass of water comes from the 1 oxygen atom hydrogen oxygen Percent Composition Practice • KNO2 Percent composition of N mass of element percent by mass x 100 mass of compound 2. % P in Al2(PO4)3 3. % Cl in LiCl 4. % Ca in CaSO4 a. 40.1 % b. 29.44 % c. 136.2 % d. 70.5 % 5. % H in NaOH a. 2.5 % b. 5.0 % c. 97.5 % d. 95 % 6. % H in (NH4)3PO4 a. 0.67 % b. 2.68 % c. 4.70 % d. 8.05 % 7. Cl in CuCl2 a. 26.39 % b. 52.79 % c. 1.89 % d. 3.79 % 8. % Sn in SnSO3 a. b. c. d. 0.60 % 59.71 % 1.67 % 167 % 9. % O in Mg(OH)2 a. 27.44 % b. 18.22 % c. 37.82 % d. 54.89 % • Calculate the percent composition – Phosphorus in Al2(PO4)3 • P = 3 x 31.0 = 93 g/mol • Al2(PO4)3 = 2 x 27 + 3 x 31 + 12 x 16 = 339 g/mol • % P = (93 / 339) x 100 = 27.43% – Chlorine in LiCl • Cl = 1 x 35.5 = 35.5 g/mol • LiCl= 1 x 6.9 + 1 x 35.5 = 42.4 g/mol • % Cl = (35.5 / 42.4) x 100 = 83.73% – Calcium in CaSO4 • Ca = 1 x 40.1 = 40.1 g/mol • CaSO4 = 1 x 40.1 + 1 x 32.1 + 4 x 16 = 136.2 g/mol • % Ca = (40.1 / 136.2) x 100 = 29.44% • Calculate the percent composition – Sodium in NaCl • Na = 1 x 23 g/mol = 23 g/mol • NaCl = 1 x 23 + 1 x 35.5 = 58.5 g/mol • % Na = (23 / 58.5) x 100 = 39.32% – Sulfur in H2SO4 • S = 1 x 32.1 = 32.1 g/mol • H2SO4 = 2 x 1 + 1 x 32.1 + 4 x 16 = 98.1 g/mol • % S = (32.1 / 98.1) x 100 = 32.72% – Potassium in KNO3 • K = 1 x 39.1 = 39.1 g/mol • KNO3 = 1 x 39.1 + 1 x 14 + 3 x 16 = 101.1 g/mol • % K = (39.1 / 101.1) x 100 = 38.67% Empirical vs Molecular Formulas • Empirical formula = formula with smallest whole number ratio of elements – Recognize because it is the simplified formula • Molecular formula = formula with actual number of atoms of each element Empirical Molecular C4H9 C8H18 H2O H6O3 We use the percent composition to calculate the empirical and molecular formulas. Which is the empirical formula? a. C3H6O2 b. C6H12O4 a. C22H24O12 b. C11H12O6 Which is the empirical formula? a. C2H6 b. CH3 c. C4H12 a. N4O12 b. N2O6 c. NO3 How to calculate empirical formula 1. Use mass of elements to calculate moles 2. Pick the smallest number of moles 3. Divide each by the smallest number of moles • • • should get whole numbers. If not, multiply to get whole numbers .25 .50 .75 .33 .66 4. Write empirical formula • A blue solid is found to contain 36.43% nitrogen and 63.16% oxygen. What is the empirical formula? • A blue solid is found to contain 36.43% nitrogen and 63.16% oxygen. What is the empirical formula? If we assume sample is 100 grams, we have 36.43 g N and 63.16 g O. 1. Calculate moles of each element N = 36.43 / 14 = 2.60 mol O = 63.16 / 16 = 3.95 mol 2. Smallest number of moles = nitrogen with 2.60 mol 3. Divide by smallest number of moles N = 2.60 / 2.60 = 1 x 2 = 2 O = 3.95 / 2.60 = 1.5 x 2 = 3 we must get whole numbers as our answer—that’s why we multiplied by two 4. Empirical formula = N2O3 • Determine the empirical formula for a compound that contains 35.98% aluminum and 64.02% sulfur • Determine the empirical formula for a compound that contains 35.98% aluminum and 64.02% sulfur If we assume sample is 100 grams, we have 35.98 g Al and 64.02 g S. 1. Calculate moles of each element Al = 35.98 / 27 = 1.33 mol S = 64.02 / 32.1 = 2.00 mol 2. Smallest number of moles = aluminum with 1.33 mol 3. Divide by smallest number of moles Al = 1.33 / 1.33 = 1 x 2 = 2 S = 2.00 / 1.33 = 1.5 x 2 = 3 we must get whole numbers as our answer—that’s why we multiplied by two 4. Empirical formula = Al2S3 • Find the empirical formula for a substance that contains 48.64% carbon, 8.16% hydrogen, and 43.20% oxygen • Find the empirical formula for a substance that contains 48.64% carbon, 8.16% hydrogen, and 43.20% oxygen If we assume sample is 100 grams, we have 48.64 g C, 8.16 g H, and 43.20 g O. 1. Calculate moles of each element C = 48.64 / 12 = 4.05 mol H = 8.16 / 1 = 8.16 mol O = 43.20 / 16 = 2.70 mol 2. Smallest number of moles = oxygen with 2.70 moles 3. Divide by smallest number of moles C = 4.05 / 2.70 = 1.5 x 2 = 3 H = 8.16 / 2.70 = 3.0 x 2 = 6 O = 2.70 / 2.70 = 1.0 x 2 = 2 we must get whole numbers as our answer—that’s why we multiplied by two 4. Empirical formula = C3H6O2 • The chemical analysis of aspirin indicates that the molecule is 60%carbon, 4.44% hydrogen, and 35.56% oxygen. Determine the empirical formula for aspirin.