cont.
advertisement

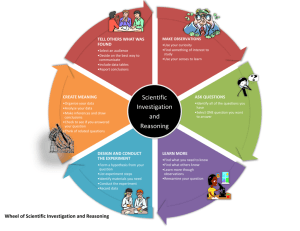
Alchemy Unit Investigation III: A Particulate World Lesson 1: Pudding and Clouds Lesson 2: Building Atoms Lesson 3: Subatomic Heavyweights Lesson 4: Life on the Edge Lesson 5: Shell Game Lesson 6: Go Figure Lesson 7: Technicolor Atoms Alchemy Unit – Investigation III Lesson 1: Pudding and Clouds ChemCatalyst In the 5th century BCE a Greek philosopher named Leucippus and his student, Democritis, stated, “All matter is made up of particles that can be divided called atoms.” • What do you think atoms are? © 2004 Key Curriculum Press. Unit 1 • Investigation III The Big Question • How have chemists thought about the atom through history? © 2004 Key Curriculum Press. Unit 1 • Investigation III You will be able to: • Describe some models of an atom and explain how they differ. © 2004 Key Curriculum Press. Unit 1 • Investigation III Notes • Atoms are extremely small particles, which cannot be seen, even with microscopes. • All matter is made up of atoms. • Scientists have created models to describe atoms. Models are similar to theories, but often include a picture or physical representation. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III (cont.) • When a model is supported by scientific evidence it is often accepted by the scientific community. • Scientific evidence is a collection of observations that many people have made. Everyone agrees on the same collection of observations. • As new evidence is gathered, models are refined and changed. © 2004 Key Curriculum Press. Unit 1 • Investigation III Activity Purpose: This lesson will introduce you to various models for the atom that have appeared over the past two hundred years. The descriptions of five models of the atom are on a separate handout. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III (cont.) Five Models of the Atom © 2004 Key Curriculum Press. Unit 1 • Investigation III Making Sense • Examine the date of the atomic evidence and then put the five models in the correct order of their introduction to the world of science. © 2004 Key Curriculum Press. Unit 1 • Investigation III Notes • An atom is mostly empty space. • The rest consists of a nucleus, which is located in the very center of the atom, and electrons, which are located around the nucleus. • The nucleus is very small (it would be nothing more than a tiny speck in our drawings, if we were to draw it to scale). (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III Notes (cont.) • The nucleus is also very dense and consists of two types of particles— neutrons and protons. • A neutron is a neutral particle with no charge on it. • A proton is a positively charged particle. • Tightly bound together, neutrons and protons make a positively charged nucleus. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III Notes (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III Check-In Here is a Bohr model of a carbon atom. • List two things this model tells you about atoms. • List something this model does not tell you about atoms. © 2004 Key Curriculum Press. Unit 1 • Investigation III Wrap-Up • All matter is made up of extremely small particles called atoms. These particles are too small to be seen even with a microscope. • Science is theoretical and dynamic. Models and theories are continually being revised, refined, or replaced with new models and theories. © 2004 Key Curriculum Press. Unit 1 • Investigation III Alchemy Unit – Investigation III Lesson 2: Building Atoms ChemCatalyst A Bohr model of a helium atom and a beryllium atom are given below. Helium, He Berylium, Be (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III (cont.) • List three similarities and three differences. • How do you think a gold atom is different from a copper atom? © 2004 Key Curriculum Press. Unit 1 • Investigation III The Big Question • What does the periodic table tells us about the structures of different atoms? © 2004 Key Curriculum Press. Unit 1 • Investigation III You will be able to: • Use the periodic table to identify the properties of an elements atom. © 2004 Key Curriculum Press. Unit 1 • Investigation III Notes • Atomic number is the number of protons in the nucleus of an atom. • Mass number is the mass of an individual atom. © 2004 Key Curriculum Press. Unit 1 • Investigation III Activity Purpose: This lesson will formally introduce you to atomic structure. Beryllium Atom Fluorine Atom Carbon Atom (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III (cont.) element chemical symbol atomic number # of protons # of electrons # of neutrons beryllium 5 fluorine 10 6 18 lead 126 19 tin 35.45 39 70 tungsten 184 29 gold atomic weight 12 chlorine potassium mass number 183.85 36 118 © 2004 Key Curriculum Press. Unit 1 • Investigation III Making Sense • If you know the atomic number of an element, what other information can you figure out about the atoms of that element? • If you know the atomic number of an element, can you figure out how many neutrons an atom of that element has? Can you come up with a close guess? Explain. © 2004 Key Curriculum Press. Unit 1 • Investigation III Notes • Mass number is the number of protons plus the number of neutrons. • Atomic mass is the “weight” or mass of a single atom. • Atomic weight is the decimal number on the periodic table. © 2004 Key Curriculum Press. Unit 1 • Investigation III Check-In Use your periodic table to identify the following elements: a) Atomic number 18 b) Has three electrons c) Atomic mass of 16.0 © 2004 Key Curriculum Press. Unit 1 • Investigation III Wrap-Up • Each successive element has one more proton than the element preceding it. • The atomic number is equal to the number of protons. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III (cont.) • The number of electrons is equal to the number of protons (as long as the atom is neutral). • The mass number is equal to the number of protons plus the number of neutrons (most of the mass is found in the nucleus). © 2004 Key Curriculum Press. Unit 1 • Investigation III Alchemy Unit – Investigation III Lesson 3: Subatomic Heavyweights ChemCatalyst A chemist investigating a sample of lithium found that some atoms have a lower mass than other atoms. The chemist determined that the structures of the two types of atoms would be similar to the two drawings below. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III (cont.) • What is different about the two atoms? • What is the atomic number of each atom? • What is the mass number of each atom? • Do you think they are both lithium atoms? Why or why not? © 2004 Key Curriculum Press. Unit 1 • Investigation III The Big Question • How do isotopes of an atom account for the atomic weight of an element? © 2004 Key Curriculum Press. Unit 1 • Investigation III You will be able to: • Predict the isotopes of an element. © 2004 Key Curriculum Press. Unit 1 • Investigation III Notes • Atoms of the same element that have different numbers of neutrons are called isotopes. • Atomic mass units (amu) are “invented” measurement units of the atomic mass. © 2004 Key Curriculum Press. Unit 1 • Investigation III Activity Purpose: In this lesson you will investigate isotopes and how they affect atomic weight. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III (cont.) boron atom 1 # protons 2 3 4 5 6 7 8 9 10 # neutrons # electrons (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III (cont.) Element Boron Chemical Symbol Atomic Number Atomic Weight # of neutrons B 5 or 6 Chlorine 17 Lithium Vanadium # of # of protons electrons 6.94 V 23 Nitrogen 7 Magnesium Argon Ar 39.9 18. 20. or 22 © 2004 Key Curriculum Press. Unit 1 • Investigation III Making Sense • Explain why the atomic weights listed in the periodic table are not usually whole numbers. © 2004 Key Curriculum Press. Unit 1 • Investigation III Notes • While the element iron is defined as being made up of neutral atoms with 26 protons and 26 electrons, not every iron atom has the same number of neutrons. • Atoms that have the same number of protons but different numbers of neutrons are called isotopes. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III Notes (cont.) • What we call the atomic weight on the periodic table is actually the average atomic mass of that element’s naturally occurring isotopes. • Isotopes have similar chemical properties in that they combine with other elements to form similar compounds. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III (cont.) • Atomic Weight is the weighted average of the atomic masses of different isotopes taking into account their abundance. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III Check-In • Predict the isotopes of carbon, C. Which isotope is more abundant? How do you know? © 2004 Key Curriculum Press. Unit 1 • Investigation III Wrap-Up • Elements may have anywhere from 2 to 10 naturally occurring isotopes. • The atomic weight of an element listed on the periodic table is actually the average mass of the naturally occurring isotopes of that element. • Isotopes have the same number of protons and electrons, but different (cont.) numbers of neutrons. © 2004 Key Curriculum Press. Unit 1 • Investigation III (cont.) • Isotopes of a single element exhibit similar properties in that they form similar compounds. • Isotopes are referred to by their mass numbers. © 2004 Key Curriculum Press. Unit 1 • Investigation III Alchemy Unit – Investigation III Lesson 4: Life on the Edge ChemCatalyst The three atoms below have similar reactivity and chemical behavior. • Where are these elements located on the periodic table? • What do you think might be responsible for their similar properties? (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III The Big Question • What accounts for the similar chemistry of elements in the same group? © 2004 Key Curriculum Press. Unit 1 • Investigation III You will be able to: • Give the number of valence electrons for an element. © 2004 Key Curriculum Press. Unit 1 • Investigation III Activity Purpose: The various physical and chemical properties of the elements can be traced to the electrons. By studying electrons further we may be able to unlock the key to creating substances similar to gold. This lesson will reveal the arrangement of electrons within atoms. © 2004 Key Curriculum Press. Unit 1 • Investigation III Making Sense • Explain how you can determine the arrangement of an element’s electrons, from the element’s position on the periodic table. © 2004 Key Curriculum Press. Unit 1 • Investigation III Notes • Bohr proposed that electrons could be found in different shells around the nucleus. • The letter “n” is referred to as the quantum number. © 2004 Key Curriculum Press. Unit 1 • Investigation III (cont.) (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III (cont.) • The outermost shell of each drawing is called the valence shell. • The valence shell contains the valence electrons. • All other electrons are considered core electrons. © 2004 Key Curriculum Press. Unit 1 • Investigation III Check-In Provide the following information for element number 34. a) The element’s name and symbol. b) The number of protons in the nucleus. c) The total number of electrons for this element. d) The number of core electrons for this element. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III (cont.) e) The number of valence electrons. f) The group number for this element. g) The names of other elements with similar chemistry. © 2004 Key Curriculum Press. Unit 1 • Investigation III Wrap-Up • Electrons occupy different shells around the nucleus of an atom. • Each electron shell can hold a specific maximum number of electrons. • The valence electrons are in the outermost electron shell of an atom. Electrons that are not valence electrons are called core electrons. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III • Elements with the same number of valence electrons have similar chemistry and are in the same group. © 2004 Key Curriculum Press. Unit 1 • Investigation III Alchemy Unit – Investigation III Lesson 5: Shell Game ChemCatalyst The two drawings show two ways of representing the electron arrangement of the element calcium, Ca. • Name at least two differences. • Name at least two similarities. 1 4 3 2 4pp 2pp 3d 2s 1s 4sp 3pp 3sp © 2004 Key Curriculum Press. Unit 1 • Investigation III The Big Question • How do electron subshells relate to the periodic table? © 2004 Key Curriculum Press. Unit 1 • Investigation III You will be able to: • Identify an element based on its electron configuration. © 2004 Key Curriculum Press. Unit 1 • Investigation III Notes • Electron shells are divided into electron subshells. © 2004 Key Curriculum Press. Unit 1 • Investigation III Activity Purpose: This lesson introduces you to electron subshells. You will explore how they are related to the periodic table. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III (cont.) 4pp 2pp 3d 2s 1s 4sp 3pp 3sp © 2004 Key Curriculum Press. Unit 1 • Investigation III (cont.) Electron configuration 1s22s1 1s22s22p3 1s22s22p63s23p5 Element nitrogen, N 1s22s22p63s23p64s23d6 1s22s22p63s23p64s23d104p2 1s22s22p63s23p64s23d104p65s24d105p4 tellurium,Te © 2004 Key Curriculum Press. Unit 1 • Investigation III Making Sense • How is the organization and structure of the periodic table related to electron subshells? (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III Notes • An electron configuration is a list of all the subshells that have electrons for a given element. The number of electrons in a subshell is specified as a superscripted number. © 2004 Key Curriculum Press. Unit 1 • Investigation III Check-In • Identify the element with the following electron configuration. 1s22s22p63s23p64s23d104p3 © 2004 Key Curriculum Press. Unit 1 • Investigation III Wrap-Up • Electron shells can be divided further into subshells, referred to as; s, p, d, f. • Each subshell can hold a specific maximum number of electrons. The s subshell can hold 2 electrons, the p subshell can hold 6, the d subshell can hold 10 electrons, and the f subshell can hold 14. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III (cont.) • The periodic table can assist us in figuring out the sequence of filling the subshells with electrons. • Chemists keep track of electrons and the subshells they are in by writing electron configurations. © 2004 Key Curriculum Press. Unit 1 • Investigation III Alchemy Unit – Investigation III Lesson 6: Go Figure ChemCatalyst Consider the following electron configuration: 1s22s22p63s23p64s23d104p4 • What element do you think is represented by this electron configuration? • How many valence electrons do you think this element has? Explain your reasoning. © 2004 Key Curriculum Press. Unit 1 • Investigation III The Big Question • Where are the valence electrons in electron configurations? © 2004 Key Curriculum Press. Unit 1 • Investigation III You will be able to: • Write electron configurations and name valence electrons for an element. © 2004 Key Curriculum Press. Unit 1 • Investigation III Activity Purpose: This activity teaches you a shorthand way to keep track of electron arrangements and how to identify valence electrons. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III (cont.) Element Electron configuration 1s22s22p3 No. of valence electrons Identity of valence electrons 2s22p3 5 1s22s22p63s23p1 1s22s22p63s23p64s23d2 4s23d2 2 or 4 1s22s22p4 Chlorine 1s22s22p63s23p64s23d104p65s1 Barium (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III (cont.) Element Electron configuration No. of valence electrons Identity of valence electrons 1s22s22p63s23p64s23d104p65s24d105p6 Silver 5s2 1 or 2 Potassium Mercury 2 6s2 Terbium 2 6s2 2,3,4,5,6 4s23d4 Chromium (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III (cont.) Element Electron configuration Valence electrons Lithium, Li [He] 2s1 2s1 Potassium, K [Ar] 4s1 4s1 Scandium, Sc [Ar] 4s23d1 4s2 3d1 Zinc, Zn [Ar] 4s23d10 4s2 © 2004 Key Curriculum Press. Unit 1 • Investigation III Making Sense • If you know the electron configuration of an element, what other information can you figure out? List at least six things. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III © 2004 Key Curriculum Press. Unit 1 • Investigation III Check-In • Write the electron configuration for bromine, Br. • Write the noble gas electron configuration for Br. • How many valence electrons does Br have? Explain. © 2004 Key Curriculum Press. Unit 1 • Investigation III Wrap-Up • The noble gases can be used as placeholders in the periodic table when writing electron configurations. • In the d-block or transition elements we find some exceptions to the rules when determining valence electrons. © 2004 Key Curriculum Press. Unit 1 • Investigation III Alchemy Unit – Investigation III Lesson 7: Technicolor Atoms ChemCatalyst The ancient alchemists heated atoms to try to change them into gold. Knowledge of atomic structure assists us in figuring out what parts of an atom can be changed. • Let’s say we started with a lithium atom and changed different parts of the atom. Look at the lithium atom below and four possible changes to it. What would we end up with in each case? (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III © 2004 Key Curriculum Press. Unit 1 • Investigation III The Big Question • What part of the atom is changed by heating? © 2004 Key Curriculum Press. Unit 1 • Investigation III You will be able to: • Predict the color of the flame produced when heating a substance. © 2004 Key Curriculum Press. Unit 1 • Investigation III Activity Purpose: In this activity you will observe evidence that atomic structure is changed when atoms are heated. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III (cont.) Safety Note: • Tie back long hair. • Keep long sleeves and clothing away from the flames. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III (cont.) Note: Do not exchange wires. For each solution, only use the wire that is already in that solution. After you use the wire, be sure to put it back in the solution from which it came. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III Substance Name Color of Flame sodium carbonate potassium nitrate copper nitrate strontium nitrate potassium chloride sodium chloride copper sulfate strontium chloride sodium nitrate potassium sulfate copper wire copper penny © 2004 Key Curriculum Press. Unit 1 • Investigation III Making Sense • The yellow color of the flame for sodium indicates that the sodium atoms changed in some way when they were heated. Consider the following possibility that the electron configuration of sodium changed from [Ne]3s1 to [Ne]4p1. What is the difference between [Ne]3s1 and [Ne]4p1? (Are the total number of electrons the same? Are the electrons in the same locations?) (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III (cont.) • Do you think gold can be made by changing the arrangement of electrons in atoms? Explain. © 2004 Key Curriculum Press. Unit 1 • Investigation III Notes Red Blue/Green strontium nitrate strontium chloride copper nitrate copper sulfate copper solid copper penny Yellow/Orange Pink/Lilac sodium carbonate sodium chloride sodium nitrate potassium nitrate potassium chloride potassium sulfate (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III Notes (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation III Check-In • Predict the colors of the flames produced when heating the following substances. Explain your thinking. Copper carbonate Magnesium sulfate © 2004 Key Curriculum Press. Unit 1 • Investigation III Wrap-Up • Electrons can move between subshells within an atom. • An energy exchange occurs when electrons move farther away from the nucleus, or when they return to their original subshell. • Moving electrons within an atom does not change the identity of the atom. © 2004 Key Curriculum Press. Unit 1 • Investigation III