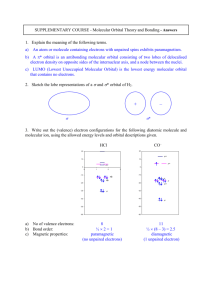
Molecular orbital
advertisement

UV-VIS Molecular Spectroscopy Chapter 13-14 From 190 to 900 nm! Reflection and Scattering Losses LAMBERT-BEER LAW Psolution P T Psolvent P0 A A a b Power of radiation after passing through the solvent Power of radiation after passing through the sample solution P log T log P0 abc kc absorptivi ty pathlength c concentrat ion Absorption Variables Beer’s law and mixtures Each analyte present in the solution absorbs light! The magnitude of the absorption depends on its e A total = A1+A2+…+An A total = e1bc1+e2bc2+…+enbcn If e1 = e2 = en then simultaneous determination is impossible Need nl’s where e’s are different to solve the mixture Assumptions Ingle and Crouch, Spectrochemical Analysis Deviations from Beer’s Law I r ( 2 1 ) 2 I 0 ( 2 1 ) 2 Successful at low analyte concentrations (0.01M)! High concentrations of other species may also affect Chemical Equilibria Consider the equilibrium: A+C AC If e is different for A and AC then the absorbance depends on the equilibrium. [A] and [AC] depend on [A]total. A plot of absorbance vs. [A]total will not be linear. Instrumental deviation with polychromatic radiation Effects of Stray Light PS stray light T P PS P0 PS A log T P PS A log P0 PS A abc kc PS 100 P0 Instrument Noise Effects of Signal-to-Noise 1 0.9 Bad at High T TRANSMISSION 0.8 0.7 0.6 0.5 0.4 0.3 0.2 Bad at Low T 0.1 0 1 2 3 4 5 6 7 8 9 10 % RELATIVE CONCENTRATION UNCERTAINTIES 11 Components of instrumentation: Sources Sample Containers Monochromators Detectors Components of instrumentation: Sources: Agron, Xenon, Deuteriun, or Tungsten lamps Sample Containers: Quartz, Borosilicate, Plastic Monochromators: Quarts prisms and all gratings Detectors: Pohotomultipliers Sources Deuterium and hydrogen lamps (160 – 375 nm) D2 + Ee → D2* → D’ + D’’ + h Excited deuterium molecule with fixed quantized energy Dissociated into two deuterium atoms with different kinetic energies Ee = ED2* = ED’ + ED’’ + hv Ee is the electrical energy absorbed by the molecule. ED2* is the fixed quantized energy of D2*, ED’ and ED’’ are kinetic energy of the two deuterium atoms. Sources Tungsten lamps (350-2500 nm) Blackbody type , temperature dependent Why add I2 in the lamps? W + I2 → WI2 Low limit: 350 nm 1)Low density 2)Glass envelope General Instrument Designs Single beam Requires a stabilized voltage supply General Instrument Designs Double Beam: Space resolved Need two detectors General Instrument Designs Double Beam: Time resolved Double Beam Instruments 1.Compensate for all but the most short term fluctuation in radiant output of the source 2.Compensate drift in transducer and amplifier 3.Compensate for wide variations in source intensity with wavelength Multi-channel Design Molar absorptivities e = 8.7 x 10 19 P A A: cross section of molecule in cm2 (~10-15) P: Probability of the electronic transition (0-1) P>0.1-1 allowable transitions P<0.01 forbidden transitions Molecular Absorption M h M* (absorption 10-8 sec) M* M heat (relaxation process) M* A+B+C (photochemical decomposition) M* M h (emission) Visible Absorption Spectra The absorption of UV-visible radiation generally results from excitation of bonding electrons. can be used for quantitative and qualitative analysis Molecular orbital is the nonlocalized fields between atoms that are occupied by bonding electrons. (when two atom orbitals combine, either a low-energy bonding molecular orbital or a high energy antibonding molecular orbital results.) Sigma () orbital The molecular orbital associated with single bonds in organic compounds Pi () orbital The molecular orbital associated with parallel overlap of atomic P orbital. n electrons No bonding electrons Molecular Transitions for UV-Visible Absorptions What electrons can we use for these transitions? MO Diagram for Formaldehyde (CH2O) H C O H = = n= Singlet vs. triplet In these diagrams, one electron has been excited (promoted) from the n to * energy levels (non-bonding to anti-bonding). One is a Singlet excited state, the other is a Triplet. Type of Transitions σ → σ* High energy required, vacuum UV range CH4: l = 125 nm n → σ* Saturated compounds, CH3OH etc (l = 150 - 250 nm) n → * and → * Mostly used! l = 200 - 700 nm Examples of UV-Visible Absorptions LOW! UV-Visible Absorption Chromophores Effects of solvents Blue shift (n- *) (Hypsocromic shift) Increasing polarity of solvent better solvation of electron pairs (n level has lower E) peak shifts to the blue (more energetic) 30 nm (hydrogen bond energy) Red shift (n- * and –*) (Bathochromic shift) Increasing polarity of solvent, then increase the attractive polarization forces between solvent and absorber, thus decreases the energy of the unexcited and excited states with the later greater peaks shift to the red 5 nm UV-Visible Absorption Chromophores Typical UV Absorption Spectra Chromophores? Effects of Multiple Chromophores The effects of substitution Auxochrome function group Auxochrome is a functional group that does not absorb in UV region but has the effect of shifting chromophore peaks to longer wavelength as well As increasing their intensity. Now solvents are your “container” They need to be transparent and do not erase the fine structure arising from the vibrational effects Polar solvents generally tend to cause this problem Same solvent must be Used when comparing absorption spectra for identification purpose. Summary of transitions for organic molecules * transition in vacuum UV (single bonds) n * saturated compounds with non-bonding electrons l ~ 150-250 nm e ~ 100-3000 ( not strong) n *, * requires unsaturated functional groups (eq. double bonds) most commonly used, energy good range for UV/Vis l ~ 200 - 700 nm n * : e ~ 10-100 *: e ~ 1000 – 10,000 List of common chromophores and their transitions Organic Compounds Most organic spectra are complex Electronic and vibration transitions superimposed Absorption bands usually broad Detailed theoretical analysis not possible, but semi-quantitative or qualitative analysis of types of bonds is possible. Effects of solvent & molecular details complicate comparison Rule of thumb for conjugation If greater then one single bond apart - e are relatively additive (hyperchromic shift) - l constant CH3CH2CH2CH=CH2 lmax= 184 emax = ~10,000 CH2=CHCH2CH2CH=CH2 lmax=185 emax = ~20,000 If conjugated - shifts to higher l’s (red shift) H2C=CHCH=CH2 lmax=217 emax = ~21,000 Spectral nomenclature of shifts What about inorganics? Common anions n* nitrate (313 nm), carbonate (217 nm) Most transition-metal ions absorb in the UV/Vis region. In the lanthanide and actinide series the absorption process results from electronic transitions of 4f and 5f electrons. For the first and second transition metal series the absorption process results from transitions of 3d and 4d electrons. The bands are often broad. The position of the maxima are strongly influenced by the chemical environment. The metal forms a complex with other stuff, called ligands. The presence of the ligands splits the d-orbital energies. Transition metal ions Charge-Transfer-Absorption A charge-transfer complex consists of an electron-donor group bonded to an electron acceptor. When this product absorbs radiation, an electron from the donor is transferred to an orbital that is largely associated with the acceptor. 1) 2) Large molar absorptivity (εmax >10,000) Many organic and inorganic complexes