Percent Composition Lesson Plan
advertisement

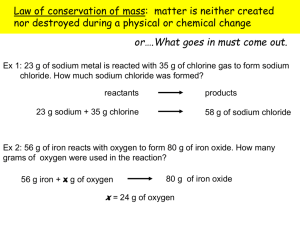
Percent Composition Lesson Plan Name: Sara Adamek Class/Subject: AP Prep Chemistry Date: March 10-15, 2014 Student Objectives/Student Outcomes: Objectives Assessment Students will be able to Students will correctly count the number of atoms. They will correctly calculate the molar mass identify which mass belongs to each atom. They will accurately multiply and of a substance. add in the correct order. Students will be able to Students will correctly determine which ratios are appropriate to use for each covert between moles, type of conversion. particles, mass and volume. Students will be able to A mole is a counting unit. It refers to 6.02 x 10^23 things. Because the define a mole and things (including atoms and compounds) have different masses moles of describe why moles of different things will have different masses. different substances have different masses. Students will be able to STP stands for Standard Temperature and Pressure. At STP all gasses have describe STP and its the same volume. This makes it easy to compare moles because the number importance for chemists. of moles is directly related to the volume. At STP 1 mole of gas takes up 22.4L. Students will be able to Take the mass of each element in the particle and divide it by the molar mass. calculate percent composition Students will be able to Students will be able to decide if a recommended serving of food fits into a apply their knowledge of specific diet. percent composition to make healthy eating decisions. Content Standards: HS-LS16. HS-PS1-7. Construct and revise an explanation based on evidence for how carbon, hydrogen, and oxygen from sugar molecules may combine with other elements to form amino acids and/or other large carbon-based molecules. Use mathematical representations to support the claim that atoms, and therefore mass, are conserved during a chemical reaction. Materials/Resources/Technology: Wks More percent composition, % comp lab Time Percent comp. intro Start of Class: Take attendance while announcements are read. Introduction of Lesson: Read up on why %comp matters and tell class Lesson Instruction: Give real examples of finding percent composition. Pass out Percent comp notes. Take notes as a class. Pass out wks and have students work in small groups. Assign groups. 1. Kristin, Cris, Erika 2. Grace, Jacob, Quinten 3. Cali, Katlyn, Jose 4. Emma, Chandler, Cory 5. Amari, Reno, Audrey 6. Brianna, Nate W. Alex B. 7. Miguel, Nate H. Alex S, Andri Assessment/Checks for understanding: Wks will be collected and checked for completion. Give feedback. Closure/Wrap-up/Review: Pass out Lab and discuss. Have students read the lab and answer the pre-lab questions. Materials/Resources/Technology: Evaporating dish iron ring Crucible tongs wire gauze Micro spatula Bunsen burner Balance goggles Ring stand CuSO4 ˖xH2 O Percent comp wks Time Percent comp lab (copper(II) sulfate hydrate lab Start of Class: Collect HW from yesterday. Get out lab. Take attendance Introduction of Lesson: Discuss the pre lab questions. SAFETY! Do not put the hot evap. dish on the table. Let it cool on the ring stand. Goggles at all times. Clean up the lab when done. Bunsen burners away, ring stands near the walls, other materials on the inside table. Lesson Instruction: Go over procedure with students. Have them repeat steps back to me. Release the students into the lab. (Groups of 2 only!) They should work on lab questions with their partner at their desks, when done with the lab. If students finish early have practice questions ready. Assessment/Checks for understanding: Each student needs to complete the lab questions. Grade for accuracy. Closure/Wrap-up/Review: Lab is due tomorrow. Discuss questions 3,6,7. Materials/Resources/Technology: Time Food labels Start of Class: Collect lab. Take attendance. Announce the exam on Friday. Pass back quiz. Remind them to study what they missed on the quiz, because it is coming back. Introduction of Lesson: Review key points of the lab (questions 4 and 5) Every day we interact with percent composition. When is that? food. Lesson Instruction: Put students in groups. 1. Grace, Cris, Erika 2. Kristin, Jacob, Quinten 3. Emma, Chandler, Cory (This group does not work) 4. Amari, Reno, Audrey (This doesn’t work for Amari) 5. Brianna, Nate W. Alex B. 6. Miguel, Andri 7. Cali, Katelyn, Jose 8. Nate H. Alex S Assessment/Checks for understanding: Grade wks and paragraph on completion. Give feedback. Closure/Wrap-up/Review: Review what % comp means. Remind them about the exam on Friday. They will need a calculator. Materials/Resources/Technology: Mole silly sentences wks. Note cards Time Group problems (silly sentences) Start of Class: Collect Hw while taking attendance. Introduction of Lesson: Remind students that there will be a test tomorrow. Today will be a review day… Lesson Instruction: Instruct students that we are going to be practicing mole conversions today. Each group needs to complete at least three of the wks (or one for each group member). Any after that point will be ec. Find something more challenging to do for the adv. students. 1. Grace, Cris, Erika 2. Kristin, Jacob, Quinten 3. Emma, Chandler, Cory 4. Amari, Reno, Audrey 5. Brianna, Nate W. Alex B. 6. Miguel, Andri 7. Cali, Katelyn, Jose 8. Nate H. Alex S Assessment/Checks for understanding: Have students check each sentence with me. Explain as necessary. No completion points. Closure/Wrap-up/Review: Remind students to look over notes and study conversions and percent compositions. Remind them to bring a calculator tomorrow. Materials/Resources/Technology: Test Time Test! Start of Class: Have students clear their desks and answer last minute questions. Introduction of Lesson: Remind them that cell phones are not allowed. When you are finished, please bring up the test and put it on the desk. There will be an e.c. wks for afterwards if you feel like it. Lesson Instruction: Pass out test. Take attendance Assessment/Checks for understanding: Grade test for accuracy. Closure/Wrap-up/Review: Wish students a good weekend. PERCENT COMPOSITION LAB Name: _____________ Pre-lab discussion: Hydrates are ionic compounds (salts) that have a definite amount of water as part of their structure. This ‘water of hydration’ is released as vapor when the hydrate is heated. The remaining solid is known as the anhydrous salt. The general reaction for heating a hydrate is: ℎ𝑦𝑑𝑟𝑎𝑡𝑒 + ℎ𝑒𝑎𝑡 → 𝑎𝑛ℎ𝑦𝑑𝑟𝑜𝑢𝑠 𝑠𝑎𝑙𝑡 + 𝑤𝑎𝑡𝑒𝑟 The percent of water in a hydrate can be found experimentally by accurately determining the mass of the hydrate and the mass of the anhydrous salt. The difference in mass is due to the water lost by the hydrate. The percent of water in the original hydrate can easily be calculated: 𝑚𝑎𝑠𝑠 𝐻2 𝑂 % 𝐻2 𝑂 = 𝑥 100 𝑚𝑎𝑠𝑠 ℎ𝑦𝑑𝑟𝑎𝑡𝑒 In this experiment, a hydrate of copper sulfate will be studied (CuSO4˖xH2O). The change from copper hydrate to anhydrous salt is accompanied by a change in color: CuSO4 ˖xH2 O (s) → CuSO4 (s) + xH2 O (g) Blue White Discussion questions: 1. Demonstrate how to calculate the percent water in you sample. Show ALL steps. 2. Based on the above reading, define anhydrous. Equipment: Evaporating dish iron ring Crucible tongs wire gauze Micro spatula Bunsen burner Balance goggles Ring stand Materials: CuSO4 ˖xH2 O Procedure: 1. Prepare the setup shown in the figure. (If your evaporating dish does not fit use wire gauze to hold it up.) 2. Find the mass of the evaporating dish. RECORD this mass. 3. With the evaporating dish on the balance, measure approximately 2 grams of CuSO4 ˖xH2 O. RECORD the exact mass. 4. Place the evaporating dish + hydrate on the wire gauze. Gently heat the dish by moving the burner back and forth around the base. Increase the heat gradually. Avoid any popping and spattering. 5. Heat strongly for 5 min or until the blue color has disappeared. During heating, a micro spatula may be used to ‘spread’ the solid and break up any ‘caked’ portions of the hydrate. Be careful not to pick up any of the solid o the micro spatula. If the edges of the solid appear to be truing brown remove the heat momentarily and resume heating at a gentler rate. 6. Allow the evaporating dish to cool until it is cool enough to be handled. Immediately find the mass of the dish and anhydrous salt, and RECORD the data. Observations and data: 1. Mass of evaporating dish _______ 2. Mass of evaporating dish and material _________ a. What color is the material? ______________ 3. Mass of evaporating dish and material after heating_______________ a. What color is the material? _______________ Analysis: (Show all work) 1. What type of reaction is this? 2. How do you know a chemical reaction happened? 3. Find the mass of the water lost. 4. What happened to the water? ‘It was lost’ is an unacceptable answer. 5. Find the percent of water in the hydrate. 6. The true value for the percent of water in this hydrate is 36.0%. Does your value agree with this value? Discuss why your values match or do not match. 7. Why do you have to weigh the anhydrous substance immediately after it cools? 8. What is the percent of Cu in the anhydrous substance? PERCENT COMPOSTION NOTES 1. What is the definition of percent composition? 2. How do you find the % composition if you know the formula? a. Calculate the percent mass of sodium in sodium chloride. 3. How do you find the percent composition if you are given masses? a. What is the percent composition of an iron chloride sample contains 13.0 g of Fe and 16.55 g of Cl. 4. What if you are given percents and grams? What do you do? a. Na3P is found to be 69.01 % Na by mass. If you have a 11.24 g samples of Na3P, what is the mass of Na in the sample? PERCENT COMPOSITION PRACTICE 1. What information do you need in order to calculate the percent mass of X in compound XY? a. Show how you would calculate percent mass of X. 2. What is the percent composition of hydrogen in H2O? 3. What is the mass percent of phosphorous in Ca3(PO4)2? 4. MgO is found to be 39.70% oxygen by mass. If you have a 15.0 g sample of MgO, what is the mass of oxygen in the sample? 5. You are given a lead oxide that is 51.8g lead and 8.0g oxygen. Determine the percent composition of each element in the compound. 6. An extra strength aspirin contains 0.50 g of aspirin. If the aspirin tablet is 0.30 g C, 0.0224 g H and 0.1776 g O, what is the percent composition of each element in aspirin? 7. Does the element with the highest subscript in a compound always have the largest percent by mass? If yes, explain. If no provide an example. NUTRITION: PERCENT COMPOSITION Names:______________ ______________ Purpose: ______________ The purpose of this activity is to explore how percent composition relates to good nutrition. Jay Leno wants to know if he is eating well. He read the National Institute of Health’s pamphlet on healthy eating and knows that to be considered healthy one’s diet should have less than 35% of the calories coming from fat. Added sugars should provide 25% or less of his calories. He needs at least 38 g of dietary fiber and should have no more than 2.3 grams of sodium. Yesterday he ate: 1.5 cups of Millville Crunchy Fruit and Nut cereal with 1 cup of skim milk 4 Chips Ahoy cookies 1 Eggplant Parmigiana ready made meal 1 can of Wild Cherry Pepsi 1 Mexican-Style chicken and vegetables ready made meal With your partners answer these questions to help Jay Leno decide if his diet is healthy or not. You will have to do your work on a separate piece of paper. Pay attention to serving size. 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. How many calories did he eat? _______________ How many calories came from fat? _________________ What percent of his calories came from fat? ____________ How many grams of sugar did he have? ______________ How may total grams of food did he eat? _________________ Hint: 1 oz= 28 g What is the percent composition of sugar in his diet? _________________ How many grams of sodium did he eat? _____________ Hint: 1 gram = 1000 mg What percent of his daily allowance of sodium did he eat? __________ How many grams of fiber did he eat? _________________ What percent of the daily recommended amount of fiber did he get? ___________ One gram of sugar provides approximately 4 calories. How many calories did he get from sugar?________ Assuming the average person is expected to eat 2,000 calories per day, what percent of calories came from sugar? ________________ According to the cereal label 5 g is 20% of the recommended daily amount of fiber. How does that compare with the government’s recommendation? When you finish the questions, write a short paragraph explaining if Jay Leno’s diet is healthy or not. Be sure to include your evidence. (Each person needs to write their own paragraph and I will be checking to make sure each is unique.) PERCENT COMPOSITION PRACTICE Name:____________________ 1. Calculate the percent mass of each element in 1 mole of the flowing compounds. a. KBr c. FeCl3 b. Na2MO4 d. Ba3(PO4)2 2. Determine the percent composition of nitrogen in each of the following compounds. a. Dinitrogen monoxide c. ammonium chloride b. Lead (II) nitrate d. potassium nitrate 3. Calcium carbonate is 12.0% carbon by mass. If a sample of calcium carbonate yields 7.35 g of carbon, what is the mass of the entire sample? 4. A compound containing cobalt is found to be 4.34 % cobalt by mass. What is the mass of the compound? 5. What is the mass percent of calcium in Ca3(PO4)2? 6. A sample of some mercury oxide was found to contain 45.2 g mercury and 15.8 g oxygen. What is the percent composition of the compound for both elements? 7. CuCl2 is found to be 34.6% copper. If I have 85.4 g of CuCl2, what is the mass of copper in that sample? MOLE REVIEW : If you can solve these problems you will have no trouble with the exam. Calculate the molar masses for each formula. 1. 2. 3. 4. 5. Carbon dioxide Hydrogen nitrate Carbon tetrachloride Sodium sulfate Argon hexafluoride What is the percent composition of each element of the following formulas? 1. 2. 3. 4. 5. Carbon dioxide Hydrogen nitrate Carbon tetrachloride Sodium sulfate Argon hexafluoride Solve the following: 1. A student prepares a compound of tungsten chloride from 3.95 g of tungsten and 3.81 g of chlorine. Assuming the reaction goes to completion, calculate the percent composition of each element. 2. A 45.9 g sample of Copper, Cu, combined with oxygen to give 51.69 g of an oxide compound. What is the percent composition of the elements? How many grams of each element go into the compound? 3. A sample of Na2S was analyzed and found to contain 567.0 g of sulfur. How much did the original compound weigh? 4. A 5.016 g sample of chromium, Cr, combined with oxygen to give 7.333 g of an oxide compound. What is the percent composition of the compound? 5. 6.04 g of Al2S3 was analyzed. What is the percent composition of S in the compound? What is the mass of S in the compound? Solve the following: 1. How many molecules are in 22.4 L of carbon dioxide gas at STP? 2. What mass does 7.45 x 1024 molecules of barium hydroxide have? 3. What is the volume of 92.19 grams of xenon gas at STP? 4. Determine the number of grams in 11.72 L of sulfur trioxide gas at STP. 5. How many molecules are in 123.45 grams of chromium (IV) nitrate? 6. Determine the number of liters in 2.11 x 1023 molecules of chlorine gas. 7. How many ions are in 106.02 grams of cobalt (II) phosphide? 8. How many atoms of iron are present in 126.90 grams of iron (III) oxide? MOLE REVIEW SOLUTIONS: Calculate the molar masses for each formula. 1. 2. 3. 4. 5. Carbon dioxide CO2 = 44.01 g/mol Hydrogen nitrate HNO3 = 63.02 g/mol Carbon tetrachloride CCl4 = 153.81 g/mol Sodium sulfate Na2SO4 = 141.87 g/mol Argon hexafluoride ArF6 = 153.95 g/mol Percent composition: 1. 2. 3. 4. 5. C 27.3% O 72.7% H 1.6%, N 22.2%, O 76.2% C 7.8%, Cl 92.2% Na 32.4%, S 22.6%, O 45.1% Ar 25.9%, F 74.1 % Solve the following: 1. 2. 3. 4. 5. 50.9% W, 49.1% Cl 88.8% Cu, 11.2% O (45.90 g Cu, 5.78g O) 962.6 g 68.4% Cr, 31.6% O 54.3% S , 3.28g S Solve the following 1. 2. 3. 4. 5. 6. 7. 8. 6.02 x 1023 molecules 2120.5 g 15.73 L 41.89 g 2.48 x 1023 molecules 7.85 L 1.34 x 1024 ions 9.57 x 1023 atoms