Biology
Slide
1 of 44
Copyright Pearson Prentice Hall
End Show
2-2 Properties of Water
Slide
2 of 44
Copyright Pearson Prentice Hall
End Show
2-2 Properties of Water
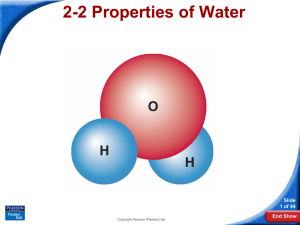
The Water Molecule
A water molecule is polar because there
is an uneven distribution of electrons
between the oxygen and hydrogen
atoms.
Slide
3 of 44
Copyright Pearson Prentice Hall
End Show
2-2 Properties of Water
The Water Molecule
Water Molecule
Slide
4 of 44
Copyright Pearson Prentice Hall
End Show
2-2 Properties of Water
The Water Molecule
Hydrogen Bonds
Because of their partial positive and negative
charges, polar molecules can attract each other.
Slide
5 of 44
Copyright Pearson Prentice Hall
End Show
2-2 Properties of Water
The Water Molecule
Cohesion is an attraction between molecules of the
same substance.
Because of hydrogen bonding, water is extremely
cohesive.
Slide
6 of 44
Copyright Pearson Prentice Hall
End Show
2-2 Properties of Water
The Water Molecule
Adhesion is an attraction between molecules of
different substances.
Slide
7 of 44
Copyright Pearson Prentice Hall
End Show
2-2 Properties of Water
Solutions and Suspensions
Solutions and Suspensions
A mixture is a material composed of two or more
elements or compounds that are physically mixed
but not chemically combined.
Slide
8 of 44
Copyright Pearson Prentice Hall
End Show
2-2 Properties of Water
Solutions and Suspensions
Two types of mixtures can be made with water
• solutions
• suspensions
Slide
9 of 44
Copyright Pearson Prentice Hall
End Show
2-2 Properties of Water
Solutions and Suspensions
Solutions
All the components of a solution are evenly
distributed throughout the solution.
solute—the substance that is dissolved.
solvent—the substance in which the solute
dissolves.
Slide
10 of 44
Copyright Pearson Prentice Hall
End Show
2-2 Properties of Water
Solutions and Suspensions
When a crystal of table salt is placed in warm water,
sodium and chloride ions are attracted to the polar
water molecules.
Cl Cl Na+
Na+
Water
Water
Slide
11 of 44
Copyright Pearson Prentice Hall
End Show
2-2 Properties of Water
Solutions and Suspensions
Suspensions
Some materials do not dissolve when placed in
water but separate into pieces so small that they
do not settle out easily.
Slide
12 of 44
Copyright Pearson Prentice Hall
End Show
2-2 Properties of Water
Acids, Bases, and pH
Acids, Bases, and pH
A water molecule is neutral, but can react to form
hydrogen and hydroxide ions.
H2O H+ + OH-
Slide
13 of 44
Copyright Pearson Prentice Hall
End Show
2-2 Properties of Water
Acids, Bases, and pH
The pH scale
Chemists devised a measurement system called
the pH scale to indicate the concentration of H+
ions in solution.
The pH scale ranges from 0 to 14.
Slide
14 of 44
Copyright Pearson Prentice Hall
End Show
2-2 Properties of Water
Acids, Bases, and pH
The pH Scale
At a pH of 7, the
concentration of H+
ions and OH- ions is
equal.
Sea water
Human blood
Pure water
Milk
Normal rainfall
Slide
15 of 44
Copyright Pearson Prentice Hall
End Show
2-2 Properties of Water
Acids, Bases, and pH
Acids
An acid is any compound that forms H+ ions in
solution.
Slide
16 of 44
Copyright Pearson Prentice Hall
End Show
2-2 Properties of Water
Acids, Bases, and pH
Bases
A base is a compound that produces hydroxide
ions (OH- ions) in solution.
Slide
17 of 44
Copyright Pearson Prentice Hall
End Show
2-2 Properties of Water
Acids, Bases, and pH
Buffers
The pH of the fluids within most cells in the human
body must generally be kept between 6.5 and 7.5.
Controlling pH is important for maintaining
homeostasis.
Copyright Pearson Prentice Hall
Slide
18 of 44
End Show
2-2
Click to Launch:
Continue to:
- or -
Slide
19 of 44
End Show
Copyright Pearson Prentice Hall
2-2
A molecule in which the charges are unevenly
distributed is called a
a. polar molecule.
b. cohesive molecule.
c. hydrogen molecule.
d. covalent molecule.
Slide
20 of 44
End Show
Copyright Pearson Prentice Hall
2-2
A dissolved substance is called a
a. solvent.
b. solution.
c. solute.
d. Suspension.
Slide
21 of 44
End Show
Copyright Pearson Prentice Hall
2-2
A compound that produces hydroxide ions in
solution is called a(an)
a. base.
b. buffer.
c. acid.
d. salt.
Slide
22 of 44
End Show
Copyright Pearson Prentice Hall
2-2
Hydrogen bonds between water molecules
result from
a. adhesion between water molecules.
b. magnetic attractions between water
molecules.
c. uneven electron distribution in each water
molecule.
d. ionic bonds in the water molecule.
Slide
23 of 44
End Show
Copyright Pearson Prentice Hall
2-2
On a pH scale, a value of 2 means that the
solution has
a. equal concentrations of H+ and OH- ions.
b. the same concentration of H+ ions as pure
water.
c. higher concentration of H+ than in pure
water.
d. lower concentration of H+ than in pure water.
Slide
24 of 44
End Show
Copyright Pearson Prentice Hall
END OF SECTION