9 - MIT
advertisement

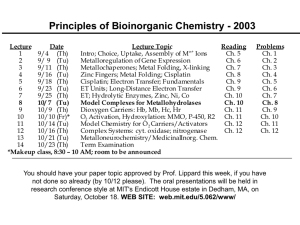
Principles of Bioinorganic Chemistry
Lecture
1
2
3
4
5
6
7
8
9
10
11
12
13
14
*Makeup
Date
Lecture Topic
Reading
n+
9/4 (Th)
Intro; Choice, Uptake, Assembly of M Ions
Ch. 5
9/ 9 (Tu)
Metalloregulation of Gene Expression
Ch. 6
9/11 (Th)
Metallochaperones; Metal Folding, X-linking
Ch. 7
9/16 (Tu)
Zinc Fingers; Metal Folding; Cisplatin
Ch. 8
9/18 (Th)
Cisplatin; Electron Transfer; Fundamentals
Ch. 9
9/23 (Tu)
ET Units; Long-Distance Electron Transfer
Ch. 9
9/25 (Th)
ET; Hydrolytic Enzymes, Zinc, Ni, Co
Ch. 10
10/ 7 (Tu)
Model Complexes for Metallohydrolases
Ch. 10
10/ 9 (Th)
Dioxygen Carriers: Hb, Mb, Hc, Hr
Ch. 11
10/10 (Fr)*
O2 Activation, Hydroxylation: MMO, P-450, R2 Ch. 11
10/14 (Tu)
Model Chemistry for O 2 Carriers/Activators
Ch. 12
10/16 (Th)
Complex Systems: cyt. oxidase; nitrogenase
Ch. 12
10/21 (Tu)
Metalloneurochemistry/MedicinalInorg. Chem.
10/23 (Th)
Term Examination
class, 8:30 – 10 AM; room 2-135
Problems
Ch. 1
Ch. 2
Ch. 3
Ch. 4
Ch. 5
Ch. 6
Ch. 7
Ch. 8
Ch. 9
Ch. 10
Ch. 11
Ch. 12
You should have your paper topic approved by Prof. Lippard this week, if you have
not done so already (by 10/12 please). The oral presentations will be held in
research conference style at MIT's Endicott House estate in Dedham, MA, on
Saturday, October 18. WEB SITE: web.mit.edu/5.062/www/
Dinuclear Metalloenzymes
Redox-active dinuclear
Metalloenzymes:
Methane monooxygenase (Fe 2)
Tyrosinase (Cu 2)
Catalase (Mn 2)
Isomerase:
Peptide hydrolases:
Xylose isomerase (Mg 2)
Phosphoester hydrolases:
Ser/Thr phosphatases (Fe/Zn or Fe/Fe)
Alkaline phosphatase (Zn 2)
Nuclease P1 (Zn 2)
Inositol Monophosphatase (Mg 2)
RNase (Mn 2 and Mg 2)
DNA polymerase I (Mg 2)
Other metallohydrolases:
Arginase (Mn 2, Co 2)
Urease (Ni 2)
-Lactamase (Zn 2)
Methionine aminopeptidase ( Zn 2 or Co 2)
Leucine aminopeptidase (Zn 2)
The Dinickel(II) Metalloenzyme Urease
History of Urease
1926, Sumner crystallizes urease
O
1975, Blakeley and Zerner discover
that urease is a dinickel enzyme
N
N
N
1995, Hausinger and Karplus determine
X-ray structure; unusual active site
O
Ni
N
N
Ni
O
O
N
Urea Hydrolysis
O
H2N
NH2
H2 O
urease
O
H2N
OH
+ NH3
NH3 + H2 O
N
O
N
N
Native and Inhibited Urease from B. Pasteurii
Lys220*
His275
HN
HN
HN
O
N
Ni 1
N
O
H
N
His137
N
Ni 2
O
H(2)
OH2
H2O
His249
N
H
O
O
His139
N
Asp363
Native urease, 2.0 Å resolution
His
HN
HN
27 5
Lys220*
HN
H
N
His137
N
O
O
N
Ni1
Ni2 N
N
S
N
HO
O
H
249
His
O Asp363
His
HN
His139
-Mercaptoethanol inhibited urease,
1.65 Å resolution
HN
27 5
Lys220*
HN
H
N
His137
N
O
O
N
Ni1
Ni2 N
O
N
O P NH O
N
2
H
His249 NH
3 63
2 O Asp
His139
DAP inhibited urease, 2.0 Å resolution
Benini et. al.Structure 1999, 7, 205-216.
Benini et. al. JBIC 1998, 3, 268-273.
Proposed Mechanism of Urea Hydrolysis
O
CO2 + (2)NH3
Ni
H2O
H2N
Ni
NH2
OH
Ni
Ni
O
Ni
O
Ni
OH
O
H2N
NH2
NH2
NH3
Other urease substrates:
O
H 2 N NHCH3
O
O
O
H 2 N NHOH HOHN NHOH H NH 2
O
NH 2
Alternative Mechanism of Urea Hydrolysis
O
CO2 + (2)NH3
Ni
H2 O
H2 N
Ni
NH2
OH
Ni
Ni
Ni
O
OH2
Ni
OH
C
O
H2 N
N
NH3
NH2
Metallo--lactamases, an Emerging Clinical Problem
PZn(OH2)2+
PZn(OH)+ + H+
R'
Reaction Catalyzed:
R'
S
C
H2 O
N
R"
O
S
C
HN
OH
H86
H 2O
Zn2
Zn1
OH
O
O
D90
Bacillus cereus
Zn...Zn, 3.5 and 4.4 Å
H210
H84
Zn1
H160
R"
COOH
C168
H149
H88
O
COOH
Active Sites:
H86
Keq = 10-7M = kf/kr
H
O
H162
D88
Zn2
H 2O
H225
H89
H101
H
O
Zn1
H99
C181
Zn2
OH2
H223
D103
S185
Stenotrophomonaso maltophilia
Zn ...Zn, 3.4 Å
Bacteroides fragilis
Zn...Zn, 3.5 Å
-Lactamase from Bacteroides fragilis
H99
D103
Wat1
H223
Zn2
Zn1
H101
Wat2
C181
H162
N.O. Concha, B.A. Rasmussen, K. Bush, O.
Herzberg (1996), Structure 4, 823-836
Active Site of a -Lactam Antibiotic
Resistance Enzyme, IMP-1 Metallo- -lactamase
(Fitzgerald, et al., 1999 )
His145
HN
Cys164
N
S
His206
HN
N
NH
Zn
Zn
O
H
O
O
Asp86
N
N
His84
His82
NH
Possible Mechanism for Metallo--lactamases
O
O
Zn
Zn
HO O
O
Zn
Zn
O
O
R1
S
OH
O
O-
N-
O
N
O
OH
NH
O
R1
R2
S
R2
S
blue intermediate
O
O
S
O
-
R2
R1
O
NH
6 S
5
4N
O
-9
COO
1
400 nm
NH
10
12
NO2
H2O
S
S
O
OH HN
COO496 nm
NO2
nitrocefin: a substrate for investigating the mechanism
NO2
NO2
Summary - Points to Remember
•Both mono- and dimetallic centers lower the pKa value of
bound water, allowing hydroxide to be delivered at pH 7.
•Coordination of the leaving group portion of the substrate
to a metal ion activates the substrate for nucleophilic attack.
•Residues not coordinated but in the second coordination
sphere can participate directly (serine in phophatases) or
indirectly (arginine in alcohol dehydrogenase) in substrate
attack, orientation, and/or activation.
•Carboxylate shifts facilitate substrate binding, activation.
•Redox inactive metal ions (Zn2+, Ni2+, Mn 2+, Co2+) preferred.
Preparation of BPAN; First Step
Functionalization of 2, 7 Positions of 1,8-Naphthyridine
+
N
O
Polyphosphoric
acid
O
NH2
100 ÞC
OEt
O
N
N
H
SeO2
350 ÞC
O
Cl
POCl3
N
N
4 atm. H2
90-130 ÞC
H
N
O
N
H
N
O
J. Heterocycl. Chem. 1982, 19, 1017-1019
Pd/CaCO3
N
HNO3
N
X
N
O
SOCl2
N
X
N
O
X = OH
X = Cl
Synthesis of BPAN, Step 2; a Naphthyridine-Based
Dinucleating Ligands for Metallohydrolase Modeling
H
N
O
H
N
O
+
2
1. MeOH
2. NaBH 4/MeOH
N
NH2
N
N
NH
HN
N
N
BPAN
Notes: The naphthyridine moiety affords a masked carboxylate
Substitution on the ring allows a convergent dinucleatin
ligand to be attained. The synthesis is high yield and ca
afford grams of the BPAN ligand.
Synthesis of [Zn2(-OH)(-Ph2PO2)(BPAN)](ClO4)2
Ph
Ph
N
N
NH
2 Zn(OTf)2 , LiPh2 PO2
NaClO4
HN
N
H2O/EtOH, pH 7.5
N
P
O
N
BPAN
Zn1 ··· Zn2 3.287 (5) Å
Zn1 ··· O1 1.949 (5) Å
Zn2 ··· O1 1.944 (5) Å
P1
O
N
Zn
N
O3
2+
O2
Zn1
Zn2
O1
[Zn 2(-OH)(-Ph 2PO2 )(BPAN)](ClO4)2
N
O
H
N
Zn
N
First structurally characterized
dizinc compound with a bridging hydroxide and a bridging
substrate analog.
The dizinc compound is
formed under neutral
conditions in water!
HPNP Transesterification Catalyzed by
[Zn2(-OH)(-Ph2PO2)(BPAN)](ClO4)2
O2N
Zn
O O
P
O O-
Ph
Ph
O
O2N
P
O
H
O
NPP
O
OH
O
HPNP
Zn
Zn
Ph
In pH ~ 7 aqueous
solution at 25 °C
O
O2N
O
P
O
O
H
O
Zn
H 2O
H
O
OH
O
Zn
P
O
H
O
O
Zn
Ph
P
O-
Rate x 10 -6 (mM/s)
7
6
Pseudo first order k = 1.6 x 10 -5 s-1
Uncatalyzed k = 2.7 x 10 -8 s-1
5
4
3
2
1
0
0
0.1
0.2
0.3
0.4
0.5
He, C., Lippard, S. J., J. Am. Chem.
Soc. (2000), 122, 184-185.
Conc. of catalyst (mM)
Good mimic of first step in alkaline phosphatase
Reminder
Metallo- -lactamases
Single polypeptide chain, 220-230 aa
Zinc(II) required for activity
Hydrolyze wide substrate range: penicillin, cephamycin
imipenem
R'
Reaction Catalyzed:
R'
S
C
H2 O
N
R"
O
C
HN
OH
H86
H 2O
Zn2
Zn1
OH
O
O
D90
Bacillus cereus
Zn...Zn, 3.5 and 4.4 Å
H210
H84
Zn1
H160
R"
COOH
C168
H149
H88
S
COOH
Active Sites:
H86
O
H
O
H162
D88
Zn2
H 2O
H225
H89
H101
H
O
Zn1
H99
C181
Zn2
OH2
H223
D103
S185
Stenotrophomonas maltophilia
Zn ...Zn, 3.4 Å
Bacteroides fragilis
Zn...Zn, 3.5 Å
Modeling Metallo--lactamases
Testing [Zn2(-OH)(-Ph2PO2)(BPAN)](ClO4 )2 as a model for the enzyme
Use of nitrocefin as a convenient substrate for kinetic studies
O
O
NH
O2N
NO2
N
O
S
OH
S
+ H2O
O
kobs
catalyst
390 nm
J. Am. Chem. Soc., 123, 6555-6563 (2001).
O
S
OH
OH
HN
O
NH
O2N
NO2
S
486 nm
Rate Law: [Zn 2(-OH)(-Ph2PO2)(BPAN)] 2+ Hydrolysis of Nitrocefin
-3
kpe, min
-1
1.6 10
-4
8 10
0
0 10
2 10-5
0
4 10-5
[Catalyst], M
Zn2L + N
[Zn2L] >> N
K
kpe =
Zn2L-N
K k2 [Zn2L]
K [Zn2 L] + 1
k2
P
k2 = 5.3 x 10-3 min -1
K = 9.4 x 103 M-1
Conditions: pH = 6.95, DMSO:H 2O = 1: 9, T=312.5 K
6 10-5
Effect of pH on Nitrocefin (N) Hydrolysis
Catalyzed by [Zn 2(-OH)(-Ph2PO2)(BPAN)] 2+
-2
2 10
Zn2 L(N)(OH2 )
-1
kpe, min
Ka
kH O
1 10-2
Zn2L(N)(OH) + H+
kOH
2
P
0
0 10
5
6
7
8
9
kpe =
kH O[H+] + kOH Ka
2
Ka + [H+]
pH
The terminal OH - ion
is the nucleophile!!
kH O = 7.5 x 10-4 min -1
2
kOH = 3.4 x 10-2 min -1
pKa = 8.7
Displacement of Ph2PO22- by Substrate
in [Zn2(-OH)(-Ph2PO2)(BPAN)]2+
31P NMR
0.01 M
complex
0.01 M complex
+ 5 equiv
penicillin G
Free 0.3 M
Na(Ph2PO2)
1:1=DMSO :D2O
1:9=DMSO:D2O
13
C NMR Evidence for Substrate Binding
2+
to [Zn2 (-OH)(-Ph 2PO2 )(BPAN)]
Cb
Ca
Cc
Penicillin
terminal
carboxylate
H
Cc
N
S
O
Cb
N
O
Penicillin G Ca OH
O
Penicillin +
-lactam
[Zn2(-OH)(-Ph2PO2)(BPAN)] 2+
Conclusion: Penicillin binds via the lactam O and carboxylate
Cd
Cc
S
Ca
Cb
Cephalothin
terminal
amide
Cc
O
H
N
Cb
O
Cephalothin +
[Zn2(-OH)(-Ph2PO2)(BPAN)] 2+
S
N
O
Cd
Ca
O
OH
Cephalothin
Conclusion: Cephalothin binds only via the carboxylate; infrared evidence rules out
binding through the lactam ring.
O
Mechanism of -Lactamase Activity for [Zn 2(-OH)(-Ph2PO2)(BPAN)] 2+
-O
O-
R"
HN
O
-O
O
N
S
R'
N
O
NH N
O
N
Zn
Zn
O
Ph
P
O
R'
O
R"
N
S
N
H2O
Ph
Ph2 PO2H
Ph2 PO2-
-O
O
N HN
O
N
Zn
Zn
N
N
R'
-O
O
HN
O
R'
S
O
N
R"
N
S
H2O
-O
O
-O
R"
O
R"
HN
O
S
R'
N
NH N
O
N
Zn
Zn
N HO
O
R'
O
O
N
R"
N
S
Evidence for an Intermediate in the Reaction of
Nitrocefin with [Zn2(-OH)(-Ph2PO2)(BPAN)]2+
O
NH
S
N
O
-3
kform = 8.5 x 10 min
kdis = 7.4 x 10-4 min-1
S
COO-
380 nm
-1
NO2
NO2
O
NH
S
630 nm (22,000 M-1 cm-1 )
No observable intermediate in
aqueous solution at pH 6.95 – 8.59
In neat DMSO intermediate is observed at 650 nm
O
S
-
N
O
Enz COO
665 nm
O
O
H
N
HN
OH
496 nm
NO2
NO2
S
NO2
COONO2
Evidence for Catalysis in the Reaction of
Nitrocefin with [Zn2(-OH)(-Ph2PO2)(BPAN)]2+
o = 9.0 x 10-8 M min-1
5 equivalents after 13.3 hours !
Conditions: pH = 7.91, T=40 oC, 1:9 = DMSO:buffer
Reminder
Urease from B. Pasteurii and Postulated Mechanism
Lys220*
His275
HN
N
O
Ni 1
N
Ni 2
His137
N
O
N
H
OH 2 (2)
O
H
H
O
249
2
His
O Asp363
CO2 + (2)NH3
H2 O
O
O
N
HN
Ni
H
N
HN
Ni
H2N NH2
OH
Ni
Ni
O
OH
NH 3
O
Accepted
O
OH 2
NH 2
Ni
H2 O
Ni
Ni
H2N
NH 2
His139
CO2 + (2)NH3
O
Ni
Native urease, 2.0 Å resolution
H 2 N NH 2
Ni
OH
Ni
Ni
O
C
N
Ni
OH
H2N
NH 3
O
NH 2
Alternative
1,4-Bis(di-2-pyridylmethyl)phthalazine;bdptz
O
N
H2NNH2
KOH
diethylene
glycol,
N
N
N
55% yield
n-BuLi
THF
O
O
HN NH
PCl5
cat DMF
Cl
Cl
N N
80% yield
N
N
N
N N
bdptz
80% yield
N
Bdptz is an Effective Dinucleating Ligand
4+
2 MnCl 2
N N
N
N
Mn
Cl
Cl
MeOH
N
Mn
N
2 Zn(OTf)2
N
CH3 CN
N
S
Fe
O
O
Ar
O
N
N
Zn
N
Zn
N N
CH 3CN
NaOBz
(NEt4 )2[Fe2 OCl6 ]
4+
+
Fe
O
Ar
N
N
S
N N
N
O
Fe
N
Barrios & Lippard, 2000
N
N N
2 Ni(OTf)2 6H2O
CH3 CN
N N
N
N
bdptz
2 Fe(OTf)2 6H2O
NaOBz, CH3 OH
2+
N
N N
N
Cl
Cl
N
Cl
O
Fe
O
Cl
N
N
N N
H2
N
N
O Ni
Ni
N
N
O
H2
OH2
H2 O
N
N
N
Amide Hydrolysis by a Dinickel(II) Complex
N
N
H
N
K1
O
Ni
Ni
N
N picolinamide
O
H2
H2 O
OH 2
N
N
H
O
N
N
N
N
N
S
+ NH3
Ni
Ni
Ni
Ni
N
N
N
N
S,
solvent
O
O
N
N
OH 2
S
O
NH 2
N
k2
N
The amidolysis of picolinamide was investigated. Spectroscopic
studies established the binding of the substrate; kinetic parameters
were obtained by quantitating the released ammonia as a function of
time.
Coordination of Picolinamide to [Ni 2(OH)(H 2O)3(bdptz)](OTs)3
[Ni2 (OH)(H2O)3(bdptz)](OTs)3
picolinamide
64
80
56
48
transmittance
transmittance
70
1591
1570
60
24
1673, CO
16
1605
1680
1640
1600
-1
1720
1560
1680
1640
1600
wavelength, cm
wavenumber (cm )
[Ni2 (OH)(H2O)3(bdptz)](OTs)3 + picolinamide
64
1591
56
48
transmittance
1720
1570
32
50
40
1707
40
40
32
1570
1642, CO
24
1720
1680
1640
1605
1600
-1
wavenumber (cm )
1560
-1
1560
Kinetics of Picolinamide Hydrolysis by
[Ni2(OH)(H2O)3(bdptz)](OTs)3
0.008
initial rate (M/hr)
initial rate (M/hr)
0.008
0.006
0.004
0.002
0
0
0.002
0.004
0.006
0.008
[Ni2 (OH)(H2 O)3 (bdptz)](OTs)3 , M
[Ni2] + picolinamide
K k [X]
kobs = 1 2
K1[X] + 1
K1
0.006
0.004
0.002
0
0
0.004
0.008
0.012
[picolinamide], M
[Ni2(picolinamide)]
K1 = 70±20 M-1
k2
products
k2 = (3.2±0.8) 10-4 min-1
0.016
Synthesis of Dinickel(II) BDPTZ Urea Complexes
methanol (1)
.
Ni(ClO4)2 6H2O + bdptz + x.s. urea acetonitrile (2)
1
2
Reactions of Dinickel(II) BDPTZ Urea Complexes
1 or 2
60 °C
acetonitrile
urea
kobs = (7.7 ± 0.5) = 10-4 h1
500 x faster than the
[Ni(terpy)(H2O)]2+
promoted rate.
Strong solution IR band
seen at 2164 cm-1
assigned to cyanate.
[Ni2(-OH)(-H2O)(bdptz)(H2O)2](OTs)3 reacts with
one equiv of NaNCO in aqueous ethanol to afford Xray quality crystals of the cyanate complex, [Ni2(OH)(-H2O)(bdptz)(-OCN)]2(OTs)4.
Structure of [Ni2(-OH)(-H2O)(bdptz)(-OCN)]2
Upon heating in aqueous
acetonitrile this complex
forms ammonia, as does
a solution of
[Ni2(bdptz)(H2O)3(OH)]3+,
demonstrating that the
cyanate is a viable
intermediate in the
hydrolysis of urea.
Postulated Mechanism for Urea Decomposition
4+
N N
N
N
H2O
Ni
H2
N
O Ni
N
O
H2
OH2
urea
cyanate complex
ammonia and water
This mechanism has implications for the hydrolysis of urea at the N
center in urease.
Conclusions from Metallohydrolase Modeling Studies
• Ligands from the XDK family can assemble dimetallics, the Co(II) form
of which can hydrolyze aminoguanidium ion as functional arginase
model. In water the complex disassembles and affords catalysis.
• With the use of naphthyridine-bridged, masked carboxylate ligands,
both terminal and bridging hydroxide units can catalyze hydrolytic
reactions. Functional models for metallo--lactamase and a phosphatase
in hand.
•The phthalazine-linked dimetallic family of complexes is extensive. The
dinickel(II) compound afford functional metallopeptidase and urease
model chemistry.
•CHALLENGE FOR THE FUTURE: Obtain dinucleating carboxylate
ligands with sufficient rigidity and steric bulk to avoid polymerization
reactions.
Dioxygen Carriers: Hb, Mb, Hc, Hr
Examples of Atom- and Group-Transfer Chemistry
PRINCIPLES:
•Both substrate binding and redox changes occur
•Coupled proton-electron transfer steps set the redox potentials
•Closely positioned redox/acid-base units work in concert
•Interactions with substrates/other proteins gate electron transf
•Two-electron transfer strategies include 2 metals, M-porphyrins
•Metal centers used to create or destroy radical species
•Changes in metal coordination spheres can facilitate allostery
•Bioinorganic chemistry of dioxygen paramount example
ILLUSTRATIONS:
•O2 Binding and Transport: hemoglobin (Hb), myoglobin (Mb),
hemocyanin (Hc), and hemerythrin (Hr)
•O2 Activation: cytochrome P-450, tyrosinase, methane
monooxygenase; dioxygenases
Properties of Protein Dioxygen
Carriers
Property
Hemoglobin Hemerythrin Hemocyanin
Metal
Fe
Fe
Cu
Ox. state of metal
in deoxy protein
Metal:O2
(II)
(II)
(I)
Fe:O2
2Fe:O2
2Cu:O2
Color, oxygenated Red
Violet-pink
Blue
Color,
Red-purple
deoxygenated
Metal coordination Porphyrin
ring
Molecular Weight 65,000
Colorless
Colorless
Protein side
chains
108,000
Number of
subunits
8
Protein side
chains
400,000 to
20,000,000
Many
4
Structure of Myoglobin
proximal side
distal side
Fe held into the
protein solely
by His imH
ring. Deoxy
structure has
Fe out of plane
of ring by 0.42
Å toward the
proximal side
of the
porphyrin.
Upon O2
binding, Fe
moves into ring
plane.
Structural and Spin State Changes upon Binding of
Dioxygen to an Iron Porphyrin Center
Deoxy Hb (T state)
Oxy Hb (R state). Hb binds 4 O2
molecules. When 2 are bound, T switches to R and makes the
next ones easier to bind.
High-spin
ferrous
Low-spin
ferric
Vibrational Spectroscopic Evidence that OxyHb and
OxyMb are Formally FeIII–O2- Species
From resonance Raman spectroscopy the O–O stretch
in oxyMb is measured to be ~ 1105 cm-1. The protein is
also diamagnetic (d5, Fe(III) and O2- couple).
Model Chemistry for Oxy Hb and Oxy Mb
The problem:
FeIIP + O2
IIP
..
Fe
FeIIIP–O2- PFeIII–O
.. FeIIP
2PFeIV=O:
PFeIII–O–FeIIIP
-oxo, “dimer”
ferryl
..
O–FeIIIP
The solutions:
Attach the porphyrin to a solid support to avoid
the bimolecular reaction; or, use low T, non-aqueous
solvents, and py or 1-MeIm complexes, but stability is
lost at - 45 °C or above. The best solution was the
construction of a sterically hindered cavity for dioxygen
binding to avoid the intemolecular chemistry leading to
the thermodynamic sink of the system, the (oxo)diiron(III) species.
Synthetic Models for OxyHb and OxyMb
(Collman)
(Baldwin)
The Cytochrome P-450 Reaction Cycle
When an axial site is available on the
iron porphyrin, dioxygen can bind
and/or be activated there. With protonmediated reductive activation of the O2
molecule, a peroxo intermediate forms
that converts to an FeIV=O species, the
ferryl ion.
The ferryl can oxidize hydrocarbons to
alcohols, epoxidize olefins, oxidize
amines to amine oxides and do related
chemistry.
P-450’s are liver enzymes necessary
for metabolism and used to convert
pro-drugs and pro-carcinogens to
their active forms.
Protoctechuate 3,4-Dioxygenase
Notes: dioxygenase vs.
monooxygenase; iron
oxidation state does not
change; iron acts as a Lewis
acid; semiradical character
of the catecholate ligand
activates it for direct OOC
attack by the dioxygen
molecule.
O
-
-
OOC
FeII
O
OH
-
+
OOC
III
HO
Fe
OH
His
- H2O
O
N
NH
HO
HO
O
III
His
Fe
O2
O
O
-
O
O
OOC
O
N
FeIII
O
O
N
NH
+H2 O
NH
His
O
O
O
HO
III
Fe
His
+-
OOC
OH
O
O
OH
O
O
N
NH
O
-
OOC
Hemocyanins - Dicopper Dioxygen Carriers
Properties:
Multi-subunit proteins, ranging in size up to 460 kDa.
Found in spiny lobsters, crayfish, and arachnids.
Deoxy Hc, colorless, dicopper(I)
Oxy Hc, blue, dicopper(II) peroxide
O–O, 745-750 cm-1 in the peroxide region, but low.
Unusual structure, first established by model chemistry:
O
Cu
Cu
O
Structure of Deoxyhemocyanin
The two Cu atoms
are held by six
terminal histidine
...
residues,
the Cu Cu
distance being 3.7 Å.
There is no obvious
bridging ligand.
Schematic Views of Deoxy and Oxy Hc
Note, Type III copper
Model Chemistry for Deoxy and Oxy Hc
Karlin model
Kitajima model
Monooxygenase Activity in Synthetic Cu2 Models
The dinuclear complex mediates insertion into the C–H bond. The
chemistry mimics that of tyrosinase.
Hemerythrins - Diiron Dioxygen Carriers
Properties:
Mono- (myo Hr) and multi- (Hr) subunit proteins.
Found in marine invertebrates.
Easily isolated protein; crystallizes after one step!!
Deoxy Hr, colorless, diiron(II)
Oxy Hr, red, diiron(III) peroxo
O–O, 844 cm-1 in the terminally bound peroxide region.
Fe–O–Fe, 486 cm-1, resonance enhanced symmetric
stretch. The asymmetric stretch
occurs at 757 cm-1.
Mixed-valent, semimet Hr, Fe(II)Fe(III): inactive.
Structure of Azidomethemerythrin
Contains a (-oxo)diiron(III)
core. Met, artificially oxidized.
An inactive form of the
protein. The azido anion
occupies the place of the
hydroperoxo anion in oxyHr.
The structure was
encountered for the first time
when the protein
crystallographers found it in
azidometmyoHr. Myo, single
subunit.
The electronic spectrum is
characteristic and a
consequence of
antiferromagnetic spin
exchange between the two
high-spin Fe(III) centers.
Chemistry at the Active Site of Hemerythrin (Hr)
Hydrophobic
Residues
(His)N
(His)N
H
O
FeII
(His)N O
N(His)
O
FeII N(His)
O
O
Asp
Glu
DeoxyHr
Diferrous
(His)N
O2
(His)N
H
O
FeIII
(His)N O
O
O
O N(His)
FeIIIN(His)
O
O
Asp
Glu
OxyHr
Diferric
Note proton-coupled electron transfer
Evidence for proton transfer comes from resonance Raman work
Early Structural Models for Methemerythrin
(-Carboxylato)diiron(III) Complexes
N
N
N
N
O
Fe
O
O
Fe
O
O
N
N
R
R
Armstrong, Lippard , N3 = HB(Pz) 3-
N
N
N
N
O
Fe
O
O
Fe
O
O
N
2+
N
R
R
Wieghardt, N 3 = Me 3TACN
These and related complexes have no site for binding of
azide or dioxygen related species such as hydroperoxide.
The syntheses exemplify spontaneous self-assembly.
The challenges are to make a site available, allow redox
chemistry to occur, and avoid polymerization to rust or
molecular ferric wheels and related complexes.
Early Structural Models for Deoxyhemerythrin
(-Carboxylato)diiron(II) Complexes
H
N
N
O
O
O
O
Fe
O
O
H
Fe
O
O
N
N
N
N
Fe
N
Fe
H
O
O
N
Fe
O
N
Fe
O
O
O
O
N
+
N
N
Wieghardt, N 3 = Me 3TACN
N
N
N
R
N
N
Fe
O
O
Fe
H
O
Fe
O
O
R
O
Fe
O
O
R
R
N
N
Hagen, N2 = Me 4en
2+
N
O
H
O
N
R
R
H
H
Tolman, Lippard , N2 = BIPhMe
N
N
H
O
N
O
R
N
N
O
N
N
N
Fe
N
R
O
O
O
O
R
N
Fe
N
2+
N
N
Ph
Kitajima, N3 = {HB(3,5- iPr2Pz) 3}-
Suzuki, Que and others N6O = HPTR
Que, N4 = TPA, TLA
None does the chemistry of the protein!
Properties of Oxy Hr, Deoxy Hr, and Models
Structure and Chemistry of Class I
Ribonucleotide Reductase R2 Protein
Reaction of the reduced
diiron(II) form of the R2 protein
with dioxygen affords a high
valent, Fe(III)Fe(IV) intermediate
designated as X. Intermediate X
is kinetically competent to
oxidize the tyrosyl residue to
afford a tyrosyl radical. This
radical in turn transfers
electrons to the R1 subunit of
the enzyme where a Cys-S–SCys cation radical forms. This
radical in turn initiates
chemistry to convert ribo- to
deoxyribonucleotides.
Oxidation of Methane in Methanotrophs
Methane monooxygenase (MMO)
Type I - Methylomonas methanica
Particulate MMO (Cu)
rod shaped
CH4
growth at 30 °C
bundled membranes
Type X - Methylococcus capsulatus(Bath)
Particulate and soluble MMO
depending on growth conditions
spherical
growth at 45 °C
bundled membranes
O2
H2O
CH3OH
NADH + H
Methanol
dehydrogenase
+
Formaldehyde
dehydrogenase
HCOOH
H2CO
Carbon assimilation
Formate dehydrogenase
Type II - Methylosinus trichosporiumOB3b
Particulate and soluble MMO
CO2
rod shaped
growth at 30 °C
paired membranes
ribulose monophosphate
pathway
Type I, Type X
serine
pathway
Type II
Methanotrophs are Used in Bioremediation of the Environment
Prince William Sound,
Alaska:
After the Exxon Valdez oil
spill, fertilizers were spread
on the beaches and natural
methanotrophs restored their
pristine beauty.
Plants recruit oil-detoxifying microbes, as discovered by scientists analyzing the
recovery of the environment in the Persian Gulf region following the 1991 Gulf War.
" In the root zone was a rich reservoir of well-known oil eating microbes...
one family of which (Arthrobacter) accounted for fully 95 percent..."
Science News, 148, 84 (August 5, 1995)
The Mineral Springs in Bath, England,
Source of Methylococcus capsulatus (Ba
The Restutive Contents of the WATER’s Concoctive Power: Solution of gaffes, chaos of Salts and mineral effluvia of
subterranean expiration. It cleanses the body from all blotches, scurvicial itchings and BREAKING OUTS
WHATSOEVER!
Components of the Methane Monooxygenase System
CH4 + O2 + H+ + NADH
FeIII
HO FeIII
CH3OH + H2O +NAD+
Hydroxylase:
251 kDa, binds O2 and CH4 substrates and
catalyzes hydrocarbon oxidation, epoxidation
B
FAD
S
Fe Fe
S
Coupling Protein:
15.9 kDa, facilitates electron transfer from
the reductase to hydroxylase and is required
for catalysis at the hydroxylase
Reductase:
38.5 kDa, binds and accept electrons from NADH and
transfers them to the diiron centers of the hydroxylase
How does it work? We discuss next time!
Principles Illustrated by these Cases
Substrate binding and redox changes occur:
•In all three cases, O2 binding is accompanied by electron
transfer from one or two metal ions to dioxygen.
Coupled proton-electron transfer steps set the potentials:
•In oxyHr a proton transfers from the bridging hydroxide to
the peroxo ligand; this step appears to block further
conversion to high-valent iron oxidase center(s).
Metal center used to create or destroy radical species:
•Occurs in ribonucleotide reductase R2 protein. Catechol
dioxygenase - Fe(III) coordination favors semiquinone form
of a bound ligand without redox reaction occurring.
Changes in metal coordination sphere facilitate allostery:
•Explains the cooperativity of O2 binding in Hb.
Important Relationships
Reversible O2 binding
•Iron porphyrin, Hb/Mb
O2 Activation
Iron porphyrin, P-450
•Dicopper center, Hc
tyrosinase
Dicopper center,
•Diiron center, Hr
Diiron center, R2, MMO
WHAT CONTROLS THE FUNCTION??