8 - MIT
advertisement

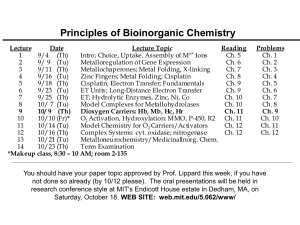
Principles of Bioinorganic Chemistry - 2003 Lecture 1 2 3 4 5 6 7 8 9 10 11 12 13 14 *Makeup Date Lecture Topic Reading n+ 9/4 (Th) Intro; Choice, Uptake, Assembly of M Ions Ch. 5 9/ 9 (Tu) Metalloregulation of Gene Expression Ch. 6 9/11 (Th) Metallochaperones; Metal Folding, X-linking Ch. 7 9/16 (Tu) Zinc Fingers; Metal Folding; Cisplatin Ch. 8 9/18 (Th) Cisplatin; Electron Transfer; Fundamentals Ch. 9 9/23 (Tu) ET Units; Long-Distance Electron Transfer Ch. 9 9/25 (Th) ET; Hydrolytic Enzymes, Zinc, Ni, Co Ch. 10 10/ 7 (Tu) Model Complexes for Metallohydrolases Ch. 10 10/9 (Th) Dioxygen Carriers: Hb, Mb, Hc, Hr Ch. 11 10/10 (Fr)* O2 Activation, Hydroxylation: MMO, P-450, R2 Ch. 11 10/14 (Tu) Model Chemistry for O 2 Carriers/Activators Ch. 12 10/16 (Th) Complex Systems: cyt. oxidase; nitrogenase Ch. 12 10/21 (Tu) Metalloneurochemistry/MedicinalInorg. Chem. 10/23 (Th) Term Examination class, 8:30 – 10 AM; room to be announced Problems Ch. 1 Ch. 2 Ch. 3 Ch. 4 Ch. 5 Ch. 6 Ch. 7 Ch. 8 Ch. 9 Ch. 10 Ch. 11 Ch. 12 You should have your paper topic approved by Prof. Lippard this week, if you have not done so already (by 10/12 please). The oral presentations will be held in research conference style at MIT's Endicott House estate in Dedham, MA, on Saturday, October 18. WEB SITE: web.mit.edu/5.062/www/ Hydrolytic Enzymes, Zinc and other Metal Ions PRINCIPLES: •M(OH)n+ centers supply OH- at pH 7 by lowering water pKa •Mn+ serves as general Lewis acid, activating substrates •Rate acceleration occurs by internal attack within coord. sphe •Protein side chains greatly assist assembly of transition state •Carboxylate shifts can occur, especially at dimetallic centers •Electrostatic interactions predominate •Non-redox active metal ions often but not universally used Illustrating the Principles: •Carboxypeptidase, carbonic anhydrase - delivering hydroxide •Alcohol dehydrogenase: an oxidoreductase •Dimetallic metallohydrolases: are two metals better than one? Carboxypeptidase A: A Hydrolytic Zinc Enzyme Reaction catalyzed: R–CH–C(O)–NH–R’ R–CH–CO2- + +NH3–R’ NH2R’’ NH2R’’ Cleaves C-terminal peptide bonds; prefers aromatic residues. Active site contains a single catalytic zinc, essential for activity. The glutamate can undergo a carboxylate shift. Thermolysin has a similar active site; it is an endopeptidase. Carboxypeptidase A structure with the inhibitor glycyl-L-tyrosine bound at the active site. Note hydrogen bonds to key residues in the active site that position the substrate moiety for bond scission. Catalytic Mechanism for Carboxypeptidase A Summary of events: 1. Substrate binds; orients by the terminal carboxylate. 2. Deprotonate bound H2O. 3. Polarize scissile bond by Arg127. 4. Bound OH- attacks peptide C(O). 5. Form tetrahedral transition state. 6.Lose 2 peptide fragments and recycle the enzyme. Principles illustrated: 1. Zinc serves as template. 2.Metal supplies cleaving reagent, OH-, and organizes key groups. 3. Chemistry achieved at neutral pH! Kcat ~ 100 s-1 . Carbonic Anhydrase, the First Known Zn Enzyme Reaction catalyzed: CO2 + H2O H2CO3 ~ 106 s-1 Carbonic Anhydrase PZn(OH2)2+ PZn(OH)+ + H+ Keq = 10-7 M = kf/kr Note: Rate 10-2 s-1 at pH 7; kf 106 s-1 in active site. Paradox: The reverse reaction is diffusion controlled, with kr ~ 1011 M-1 s Thus kf ≤ 104 s-1. So how can the turnover be 106 s-1 ? Answer: Facilitated diffusion of protons by buffer components bound to the enzyme. Possible Carbonic Anhydrase Mechanism Alcohol Dehydrogenase, an Oxidoreductase Reaction catalyzed: RCH2 OH + NAD+ RCHO + NADH + H+ Enzyme contains two 40 kDa polypeptides, each with 2 Zn2+centers in separate domains. One zinc is structural, the other catalytic. Catalytic zinc is 20 Å from the surface, near the nicotinamide binding region. This center is not required for NAD + cofactor binding. Alcohol substate DO require zinc and bind directly to the metal center, displacing the coordinated water. Schematic Diagram NAD+ binding to the active site of LADH, with specific, wellpositioned amino acid side chains holding it in place. Ethanol is shown bound to the zinc, displacing water. The system is set to undergo catalysis. Hydride Transfer Mechanism R' + H N R Zn H H2 N O O H2 O R' .. N H + RCHO + H H2 N O Zn H2 O Note hydride transfers from a-C of alcohol to nicotinamide ring. Dinuclear Metalloenzymes Redox-active dinuclear Metalloenzymes: Methane monooxygenase (Fe 2) Tyrosinase (Cu 2) Catalase (Mn 2) Isomerase: Peptide hydrolases: Xylose isomerase (Mg 2) Phosphoester hydrolases: Ser/Thr phosphatases (Fe/Zn or Fe/Fe) Alkaline phosphatase (Zn 2) Nuclease P1 (Zn 2) Inositol Monophosphatase (Mg 2) RNase (Mn 2 and Mg 2) DNA polymerase I (Mg 2) Other metallohydrolases: Arginase (Mn 2, Co 2) Urease (Ni 2) -Lactamase (Zn 2) Methionine aminopeptidase ( Zn 2 or Co 2) Leucine aminopeptidase (Zn 2) Alkaline Phosphatase; a Dizinc(II) Center Activates the Substrat 1. 3. 2. 1. The substrate binds to the dizinc center; a nearby Arg also helps activate it. 2. A serine hydroxyl group attack the phosphoryl group, cleaving the ester. The phosphate is transferred to the enzyme, forming a phosphorylserine residue. 3. Hydrolysis of this phosphate ester by a zincbound hydroxide completes the catalytic cycle. This mechanism is supported by studies with chiral phosphate esters (ROP18O17O16O)2-; there is no net change in chirality at phoshorus. pKa Values of Metal-Bound Water for Common Metal Ions in Aqueous Solution M OH2 pKa M OH + H+ Fe3+ -OH Cu2+ -OH pK a Dimetallics can move the value into the physiological range near pH 7 Zn2+ -OH Co2+ -OH Ni2+ -OH Mn2+ -OH Mg2+ -OH 1 2 3 4 5 6 7 8 9 10 11 Barnum, D. W. Inorg. Chem. 1983, 22, 2297. 12 13 14 pH Advantages of Carboxylate-Bridged Dimetallic Centers in Chemistry and Biology Modes of Substrate (S) Attack by an Activated Nucleophile (N) at a Carboxylate-Bridged Dimetallic Center O O C R MB MA B O C R O N S MA MB O C O C R : MB MA A S : : S N : :N N MB MA O D C R S N S MB MA O O E O C R Carboxylate Ligation in Metalloproteins and the Carboxylate Shift Biologically available carboxylates: O - O O - O O C C H 2C OH Carbonate - H3N C COO H2 C - H Aspartate (Asp) D Carbonate is encountered in transferrin Lys* is found in urease and rubisco H3 N C O - O O C C CH2 NH H2 C CH2 COO- H H2 C Glutamate (Glu) E H3 N CH2 C COO- H Lys* Carbamate Chemistry Leading to the Definition of the Carboxylate Shift methanol M(O2CCH 3)2 + BIPhMe M = Mn, Fe R R C C O O R O C N O O O N M M M N O O O N C O R O O C C R R [M3II (O2CCH 3)6(BIPhMe)2] "This movement, which we term 'the carboxylate shift,' may be of general importance in the active site structures of metalloproteins." Rardin, Bino, Poganiuch, Tolman, Liu, Lippard Angew. Chem. Int. Ed. Engl., 102, 812 (1990) R O O M R M O O M M R O M M O The Expanded Carboxylate Shift C O M O M M O • • C O M C O O C O R M M M M M M • • M R O C O O M R O M O R C M M O C O • • R C O M O M R M C O • • R R R O • • M C O O • • R M Note syn/anti lone pair traversals The Active Sites of Selected Dinuclear Metalloenzymes Catalyzing Hydrolysis of Biological Substrates Asn150 Asp45 H2N H2O Fe N HN O Asp90 O His281 H O H2O His92 O Zn O Trp1 O N N O Asp118 NH2 NH O Zn N O His6 HN His199 N H O H O His60 N Zn NH N O N H His116 Asp122 Nuclease P1 Calcineurin Asp301 His97 O His201 HN N N His230 HN O His57 H O Zn Zn O N NH N N O Glu152 HN O N H Asp179 His55 O O H O Zn O Zn O HN Lys169 Phosphotriesterase O N N H Asp117 Aminopeptidase His156 Structure and Chemistry of Arginase NH 2+ + H3 N CO2- N H NH 2 +H N 3 + H2 O CO2L-Ornithine L-Arginine O + H2 N Urea Asp-232 His O Asp O HN N O Mn + O Asp-128 NH 3+ O 2 H O Asp-124 O Mn Active Site of Arginase O 2+ N O His NH NH 2 Postulated Catalytic Mechanism for Arginase O NH 2 N H Mn2+ I O O O NH 2 O NH 2 O O H Mn2+ O O O N NH 2 H O Mn2+ Mn2+ H O II Asp 128 Asp 124 NH 2 + H L-Ornithine H2O L-Arginine Mn2+ H2 O O NH 2 Mn2+ Mn2+ O O NH 2 Mn2+ Christianson, 1996 III IV Urea Principles illustrated: the dimetallic affords hydroxide; the substrate is positioned by residues in the active site; the dimetallic stabilizes the urea leaving group; redox inactive metal; The Dinickel(II) Metalloenzyme Urease History of Urease 1926, Sumner crystallizes urease O 1975, Blakeley and Zerner discover that urease is a dinickel enzyme N N O N Ni 1995, Hausinger and Karplus determine X-ray structure; unusual active site N N O Ni O N Urea Hydrolysis O H2N NH2 H2 O urease O H2N OH + NH3 NH3 + H2 O N O N N Native and Inhibited Urease from B. Pasteurii Lys220* His275 HN HN HN O N Ni 1 N O H N His137 N Ni 2 O H(2) OH2 H2O His249 N H O O His139 N Asp363 Native urease, 2.0 Å resolution His HN HN 275 Lys220* HN H N His137 N O O N Ni1 Ni2 N N S N HO O H 249 3 His O Asp 63 His HN His139 -Mercaptoethanol inhibited urease, 1.65 Å resolution HN Lys220* 275 HN N O Ni1 O N H N His137 Ni2 N O N O P NH O N 2 H His249 NH 363 Asp 2 O His139 DAP inhibited urease, 2.0 Å resolution Benini et. al.Structure 1999, 7, 205-216. Benini et. al. JBIC 1998, 3, 268-273. Proposed Mechanism of Urea Hydrolysis O CO2 + (2)NH3 Ni H2O H2N Ni NH2 OH Ni Ni O Ni O Ni OH NH2 O H2N NH2 O O NH3 Other urease substrates: O H 2 N NHCH3 O H 2 N NHOH HOHN NHOH H NH 2 O NH 2 Alternative Mechanism of Urea Hydrolysis O CO2 + (2)NH3 Ni H2 O H2 N Ni NH2 OH Ni Ni Ni O OH2 Ni OH C O H2 N N NH3 NH2 Metallo--lactamases, an Emerging Clinical Problem PZn(OH2)2+ PZn(OH)+ + H+ R' Reaction Catalyzed: R' S C H2 O N R" O S C HN OH H86 H2O Zn2 Zn1 OH O O D90 Bacillus cereus Zn...Zn, 3.5 and 4.4 Å H210 H84 Zn1 H160 R" COOH C168 H149 H88 O COOH Active Sites: H86 Keq = 10-7M = kf/kr H O H162 D88 Zn2 H2O H225 H89 H101 H O Zn1 H99 C181 Zn2 OH2 H223 D103 S185 Stenotrophomonaso maltophilia Zn ...Zn, 3.4 Å Bacteroides fragilis Zn... Zn, 3.5 Å -Lactamase from Bacteroides fragilis H99 D103 Wat1 H223 Zn2 Zn1 H101 Wat2 C181 H162 N.O. Concha, B.A. Rasmussen, K. Bush, O. Herzberg (1996), Structure 4, 823-836 Active Site of a -Lactam Antibiotic Resistance Enzyme, IMP-1 Metallo- -lactamase (Fitzgerald, et al., 1999 ) His145 HN Cys164 N S His206 HN N NH Zn Zn O H O O Asp86 N N His84 His82 NH Possible Mechanism for Metallo--lactamases O O Zn Zn HO O O Zn Zn O O R1 S OH O O- N- O N O OH NH O R1 R2 S R2 S blue intermediate O O S O - R2 R1 O NH 6 S 5 4N O -9 COO 1 400 nm NH 10 12 NO2 H2 O S S O OH HN COO496 nm NO2 nitrocefin: a substrate for investigating the mechanism NO2 NO2 Summary - Points to Remember •Both mono- and dimetallic centers lower the pKa value of bound water, allowing hydroxide to be delivered at pH 7. •Coordination of the leaving group portion of the substrate to a metal ion activates the substrate for nucleophilic attack. •Residues not coordinated but in the second coordination sphere can participate directly (serine in phophatases) or indirectly (arginine in alcohol dehydrogenase) in substrate attack, orientation, and/or activation. •Carboxylate shifts facilitate substrate binding, activation. •Redox inactive metal ions (Zn2+, Ni2+, Mn 2+, Co2+) preferred.