Study Guide
advertisement

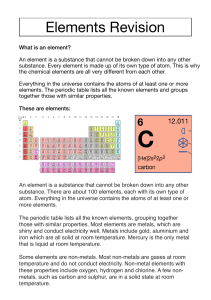
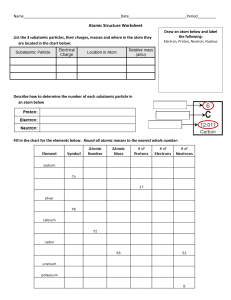
Physical Science First Semester Study Guide Name _______________________________________ 1. Know lab safety rules. Example: What should you do with chemicals that you are finished working with? _______________________________________________________________________________ 2. List and define the parts of the scientific method. Be able to apply these terms to situations. ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ 3. Define and identify the following: Observation-_________________________________________________________________________ Bias-________________________________________________________________________________ Control-_____________________________________________________________________________ Dependent variable-___________________________________________________________________ Independent variable-__________________________________________________________________ Model-______________________________________________________________________________ 4. Describe a scientific theory. Be able to answer questions regarding this term. ____________________________________________________________________________________ ____________________________________________________________________________________ 5. Describe a scientific law. _______________________________________________________________ ____________________________________________________________________________________ 6. Know which unit of measurement is used for different quantities. Example: mass is measured in _____ 7. Know which tools are used to measure certain quantities. Example: What tool is used to measure mass? ________________________________________ volume? _____________________________ temperature? ______________________ 8. Know how to convert metric prefixes. Examples: 321 cm = _________ m and 2631 g = ___________ kg. 9. Define density. ___________________________________________________________________ 10. Calculate density. Example: A substance has a mass of 300 g and a volume of 5 cm 3. What is its density? ___________________ 11. Be able to interpret data tables. 12. What are the three types of graphs and when are they most useful? Be able to interpret graphs. ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ 13. Describe and give examples of physical and chemical properties and explain how they are useful. ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ 14. Define and give examples of physical changes. ______________________________________________ ____________________________________________________________________________________ 15. Define and give examples of chemical changes. _____________________________________________ ____________________________________________________________________________________ 16. Describe types of evidence of a chemical reaction. ___________________________________________ ____________________________________________________________________________________ 17. Contrast properties of a solid, liquid, and gas. ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ 18. Define and identify the following terms. Give an example of each. Atom-_______________________________________________________________________________ Substance-___________________________________________________________________________ Element-____________________________________________________________________________ Compound-__________________________________________________________________________ Homogeneous mixture-________________________________________________________________ Heterogeneous mixture-_______________________________________________________________ Solution-____________________________________________________________________________ Colloid-______________________________________________________________________________ Suspension-__________________________________________________________________________ 19. Describe how you would separate a mixture of salt and sand. _________________________________ ___________________________________________________________________________________ 20. Describe how and why distillation can be used to separate solutions of miscible liquids. ____________________________________________________________________________________ ____________________________________________________________________________________ 21. Describe Dalton’s atomic theory. _________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ 22. Describe how the model of the atom has changed over time. Put the following scientists’ models in sequential order and describe each model. Bohr, Dalton, Rutherford, Schrodinger, Thomson ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ 23. Define atomic number. _________________________________________________________________ 24. Define mass number. __________________________________________________________________ 25. Describe average atomic mass. __________________________________________________________ ____________________________________________________________________________________ 26. Describe how electrons are arranged in atoms, specifically metals and nonmetals. ____________________________________________________________________________________ ____________________________________________________________________________________ 27. Put the following in order from smallest to largest: atom, molecule, proton, quark. ____________________________________________________________________________________ 28. Compare and contrast the subatomic particles. ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ 29. Describe an atom’s nucleus. _____________________________________________________________ ____________________________________________________________________________________ 30. What is the charge of an atom and why? __________________________________________________ ____________________________________________________________________________________ 31. Who is credited with the development of the periodic table? __________________________________ 32. The order of elements in the periodic table is based on _______________________________________ ____________________________________________________________________________________ 33. Describe the location of metals, nonmetals, and metalloids on the periodic table. ____________________________________________________________________________________ ____________________________________________________________________________________ 34. List and define the properties of metals. ___________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ 35. Describe the alkali metals. ______________________________________________________________ ____________________________________________________________________________________ 36. Describe the alkaline earth metals. _______________________________________________________ ____________________________________________________________________________________ 37. Describe the halogens. _________________________________________________________________ ____________________________________________________________________________________ 38. Describe the noble gases. _______________________________________________________________ ____________________________________________________________________________________ 39. Atoms in the same group have the same number of _________________________________________. 40. What determines whether an atom will form a positive or negative ion? _________________________ ____________________________________________________________________________________ 41. Describe what happens to sodium and chlorine atoms when they combine to form sodium chloride. ____________________________________________________________________________________ ____________________________________________________________________________________ 42. Define ionization energy. _______________________________________________________________ 43. What are the general trends for ionization energy across a period and down a group? ____________________________________________________________________________________ 44. Be able to write formulas for compounds whose names include prefixes. Example: dinitrogen tetroxide has the formula ____________________. 45. Be able to write formulas for compounds when given the ions that combine. Example: Ca2+ and Cl1-. ____________________ 46. Be able to balance equations. A chemical equation is balanced by changing or adding ______________. 47. Apply the law of conservation of mass to chemical reactions. 48. Define and identify reactants and products. ________________________________________________ ____________________________________________________________________________________ 49. Describe and identify a synthesis, decomposition, single replacement, and double replacement reaction. ____________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ 50. Describe a neutralization reaction. What is a salt? ___________________________________________ ____________________________________________________________________________________