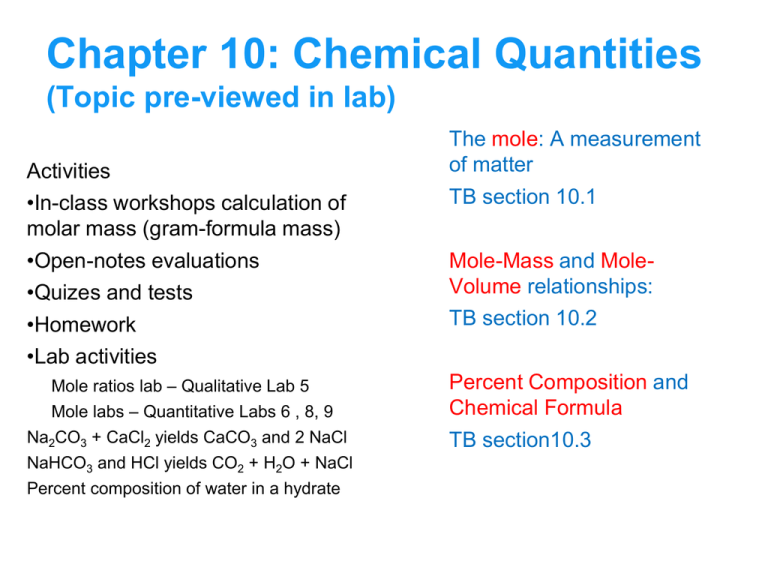
Chapter 10: Chemical Quantities
(Topic pre-viewed in lab)
Activities
•In-class workshops calculation of
molar mass (gram-formula mass)
•Open-notes evaluations
•Quizes and tests
•Homework
•Lab activities
Mole ratios lab – Qualitative Lab 5
Mole labs – Quantitative Labs 6 , 8, 9
Na2CO3 + CaCl2 yields CaCO3 and 2 NaCl
NaHCO3 and HCl yields CO2 + H2O + NaCl
Percent composition of water in a hydrate
The mole: A measurement
of matter
TB section 10.1
Mole-Mass and MoleVolume relationships:
TB section 10.2
Percent Composition and
Chemical Formula
TB section10.3
10.1
The Mole: A Measurement of> What is a Mole?
Matter
Converting Number of Particles to Moles
One mole (mol) of a substance is 6.02 1023
representative particles of that substance and is
the SI unit for measuring the amount of a
substance. The term representative particle
refers to the species present in a substance:
usually atoms, molecules, or formula units.
The number of representative particles in a mole,
6.02 1023, is called Avogadro’s number.
Slide
2 of 43
© Copyright Pearson Prentice Hall
End Show
10.1
The Mole: A Measurement of> What is a Mole?
Matter
Slide
3 of 43
© Copyright Pearson Prentice Hall
End Show
SAMPLE PROBLEM 10.2
Slide
4 of 43
© Copyright Pearson Prentice Hall
End Show
SAMPLE PROBLEM 10.2
Slide
5 of 43
© Copyright Pearson Prentice Hall
End Show
10.1
The Mole: A Measurement of> What is a Mole?
Matter
Converting Moles to Number of Particles
Slide
6 of 43
© Copyright Pearson Prentice Hall
End Show
10.3
Read the question CAREFULLY. What do you need to find?
Hints:
(1)How many molecules of propane are in one mole of
propane?
(2)How many atoms are there in one molecule of propane?
Slide
7 of 43
© Copyright Pearson Prentice Hall
End Show
SAMPLE PROBLEM 10.3
Slide
8 of 43
© Copyright Pearson Prentice Hall
End Show
SAMPLE PROBLEM 10.3
Slide
9 of 43
© Copyright Pearson Prentice Hall
End Show
SAMPLE PROBLEM 10.3
Slide
10 of 43
© Copyright Pearson Prentice Hall
End Show
Practice Problems for Sample Problem 10.3
Slide
11 of 43
© Copyright Pearson Prentice Hall
End Show
10.1
The Mole: A Measurement of> The Mass of a Mole of an
Matter
Element
The Mass of a Mole of an Element (use Periodic Table)
How is the atomic mass of an element
related to the molar mass of an element?
The atomic mass of an element expressed in
grams is the mass of a mole of the element. The
mass of a mole of an element is its molar mass
(gram-formula mass).
Slide
12 of 43
© Copyright Pearson Prentice Hall
End Show
10.1
The Mole: A Measurement of> The Mass of a Mole of an
Matter
Element
One molar mass of carbon, sulfur, mercury, and
iron are shown.
Slide
13 of 43
© Copyright Pearson Prentice Hall
End Show
10.1
The Mole: A Measurement of> The Mass of a Mole of a
Matter
Compound
The Mass of a Mole of a Compound
How is the mass of a mole of a
compound calculated?
To calculate the molar mass of a
compound, find the number of grams of
each element in one mole of the
compound. Then add the masses of the
Slide
elements in the compound.
14 of 43
© Copyright Pearson Prentice Hall
End Show
10.1
The Mole: A Measurement of> The Mass of a Mole of a
Matter
Compound
One SO3 molecule has a mass of 80.1 amu.
Substitute the unit grams for atomic mass units.
Thus 1 mol of SO3 has a mass of 80.1 g. One
mole of SO3 molecules has a mass of 80.1 grams
Slide
15 of 43
© Copyright Pearson Prentice Hall
End Show
10.1
The Mole: A Measurement of> The Mass of a Mole of a
Matter
Compound
Molar Masses of Glucose, Water, and
Paradichlorobenzene
Slide
16 of 43
© Copyright Pearson Prentice Hall
End Show
SAMPLE PROBLEM 10.4
Slide
17 of 43
© Copyright Pearson Prentice Hall
End Show
10.1 Section Quiz.
Assess students’ understanding
of the concepts in Section 10.1.
Continue to:
-or-
Launch:
Section Quiz
Slide
18 of 43
© Copyright Pearson Prentice Hall
End Show
10.1 Section Quiz.
2. A mole of hydrogen gas, H2(g), contains 6.02
x 1023
a. molecules.
b. atoms.
c. amu.
d. grams.
Slide
19 of 43
© Copyright Pearson Prentice Hall
End Show
10.1 Section Quiz.
3. The atomic mass of fluorine is 19.0 amu, so
the molar mass is
a. 19.0 amu.
b. 19.0 g.
c. 6.02 x 1023 amu.
d. 6.02 x 1023 g.
Slide
20 of 43
© Copyright Pearson Prentice Hall
End Show
10.1 Section Quiz.
4. Calculate the molar mass of ammonium nitrate.
a. 45.02 g
b. 80.05 g
c. 60.06 g
d. 48.05 g
Slide
21 of 43
© Copyright Pearson Prentice Hall
End Show





