Water, pH, buffers
advertisement

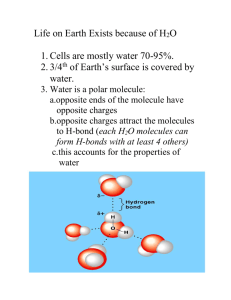
Ch.3 Life on Earth is Aqueous! Predominance of Water -3/4 of earth covered with water (liquid & solid) -cells are 70-95% water -all organisms require water for survival ~ 1 week survival time for human without water! LE 3-2 Hydrogen bonds Key properties of water defines behavior -Polarity: partial positive and negative charges -Hydrophilic nature: attracted to other water molecules and charged particles • Four of water’s properties - Cohesive behavior - Ability to moderate temperature - Expansion upon freezing - Versatility as a solvent LE 3-3 >100 ft Cohesion & Adhesion During Transpiration Water-conducting cells 100 µm H2O Cohesion Water molecules hold together through H-bonds to other water molecules Example Cohesion helps transport water against gravity in plants from roots to stems during transpiration Adhesion Water’s attraction to other charged surfaces Example Water’s attraction to cell walls helps upward transport against gravity Surface Tension: Strong ordered film-like structure at interface of water and atmosphere Held together through H-bonds Strength creates surface for small organisms to move across High specific heat 1 cal/g/oC Amount of heat gained or lost to change the temperature of 1g of water by 1ºC Compare to alcohol: specific heat of 0.6 cal/g/oC • Consequence – Lessens temperature fluctuations to within limits that permit life – Heat is absorbed to hydrogen bonds break – Heat is released when hydrogen bonds form Evaporative Cooling – transformation of a substance from liquid to gas – Heat of vaporization • The amount of heat 1 g of liquid must absorb to be converted to gas (water: ~580 cal/g at 25oC) • remaining surface cools during evaporation, a process called evaporative cooling • Consequence • Evaporative cooling of water helps stabilize temperatures in organisms and bodies of water • Perspiration: sensation? Solid water (ice): less dense than liquid because H-bonds more stable and ordered; expansion occurs Consequence: Ice floats on liquid water Insulates; prevents temperature fluctuations Example: ponds and lakes in wintertime aquatic organisms survive in the liquid water beneath ice Polar solvent Dissolves other polar or charged solutes Examples: salts, polar proteins, nucleic acids Creates an aqueous solution -through Hydration shells H-bonds LE 3-6 Hydration shells form around cations and anions – Na+ Causes salt crystals to dissolve In H2O + + – – + – – Na+ – + + Cl– Cl– + – + + – – – – LE 3-7a Lysozyme molecule (protein) in a nonaqueous environment. LE 3-7b Can you deduce what regions on lysozyme are positive and negative? Lysozyme molecule in a aqueous environment. Concepts of Hydrophilic and Hydrophobic • Hydrophilic substance – Attracted to water due to charged or polar nature e.g. salts (ionic) • Hydrophobic substance -Repelled by water due to nonpolar nature e.g. oils, fats (nonpolar) Important when considering the plasma membrane. Aqueous chemistry in biological systems • Most biochemical reactions occur in water • Most reactions are highly sensitive to pH Enzyme Reactant-1 + Reactant-2 Product What is pH and how does it relate to water? LE 3-UN53 Water occasionally produces protons (H+) and hydroxide ions (OH-) Hydronium ion (H3O+) Simplified to H+ Hydroxide ion (OH–) Water Dissociation -Hydrogen involved in H-bonds in H2O can lose electron -H+ (proton) can bond with another H2O molecule Results: – molecule with the extra proton is now a hydronium ion (H3O+) – The molecule that lost the proton is now a hydroxide ion (OH-) • Dissociation of water molecules • Rare in pure water (25oC) » [H+]=10^-7 M » [OH-]=10^-7 M • Changes in concentrations of H+ and OH- drastically affect the chemistry of a cell Such changes alter the pH pH -reflects the molar concentration of H+ in a solution pH= -log[H+] -increases in [H+] increase acidity e.g. HCl (hydrochloric acid) readily dissociates into H+ and Cl-increases in [OH-] raises alkalinity, decreases in acidity e.g. the base NaOH (sodium hydroxide) readily dissociates into Na+ and OH- The pH Scale • pH 7 occurs when [H+] =[OH-] • Acidic solutions pH < 7, [H+] > [OH-] • Basic solutions pH > 7, [H+] < [OH-] • Most biological fluids: pH 6-8 LE 3-8 pH Scale 0 Increasingly Acidic [H+] > [OH–] 1 Neutral [H+] = [OH–] Battery acid 2 Digestive (stomach) juice, lemon juice 3 Vinegar, beer, wine, cola 4 Tomato juice 5 Black coffee Rainwater 6 Urine 7 Pure water Human blood 8 Increasingly Basic [H+] < [OH–] Seawater 9 10 Milk of magnesia 11 Household ammonia 12 Household bleach 13 Oven cleaner 14 Calculating pH Given: pH= -log[H+] Constant: Water ion product 10^-14 M^2= [H+][OH-] What is the pH of a solution containing 10^-7 M H+? 10^-4 M For the same solutions, what is the concentration of OH-? Determine the concentration of H+ and OH- at pH 3. Apparent small changes in pH value are really LARGE Exponential! Calculate the difference between pH 7 and pH 4 [H+] is10^3 x larger Buffers • pH of most living cells must remain close to pH 7 • Buffers minimize changes in [H+] and [OH- ]in a solution • Most buffers consist of an acid-base pair that reversibly combines with H+ • • H CO 2 3 carbonic acid HCO - + H+ 3 bicarbonate The Damage of Acid Precipitation • Acid precipitation refers to rain, snow, or fog with a pH lower than 5.6 • Caused by the mixing of different pollutants with water in the air e.g. sulfur and nitrogen oxides • Main source: combusted fossil fuels • Acid precipitation can damage life in lakes and streams – Leaches geological buffers from soils – Solubilizes toxic heavy metals e.g. aluminum LE 3-9 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 More acidic Acid rain Normal rain More basic