Presenting

Chapter 1
Metric Units
PS 12:1
PS 12:2
SI Units of Measurement
If I tell you that I have 11, what do you know?
For a measurement to make sense, it requires both a # and a unit .
EX: I have 11 brothers/sisters.
You should always express measurements in
# ’ s and a unit so that their meaning is clear .
Many units that you are familiar with are not units used in science( feet, inches, Fahrenheit)
SI Continued
Scientists use a set of measuring units called SI, or the
International System of Units
This is a revised version of the metric system.
By everyone using the SAME system of units, scientists can easily interpret one another ’ s measurements.
7 Base Units
The seven are listed on page 16
Length – meter (m)
Mass – gram (g)
Volume – Cubic meter (m 3 ) = Liter(L)
Length: straight-line distance between 2 points
Mass: quantity of matter in an object/sample
Volume: amount of space taken up by an object
Density – kg/m 3
Density: the ratio of an object ’ s mass to its volume
Density = Mass = Kg
Volume m 3
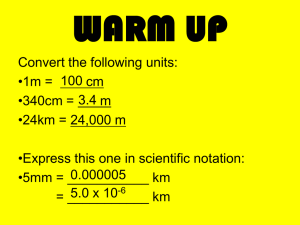
Now let ’ s practice a little with converting within SI kids hate dances because dj ’ s cruddy music k,h,da,base,d,c,m
Kilo., hecta, deca., base, deci., cent., milli.
Worksheet practice
EX: 5kg = _____ g
5.0kg = ______g
Move the decimal 3 spaces to the right to get to the base unit gram
K,ha,da,base(gram is the base unit)
Answer:
5kg = 5000g
Use this ex and complete the worksheet
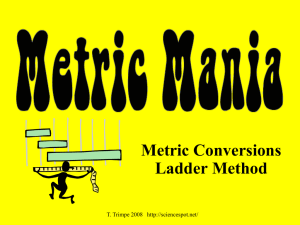
Metric Conversions
Ladder Method
T. Trimpe 2008 http://sciencespot.net/
KILO
1000
Units
1
Ladder Method
2
HECTO
100
Units
DEKA
10
Units
3
DECI
0.1
Unit
Meters
Liters
Grams
CENTI
0.01
Unit
MILLI
0.001
Unit
How do you use the “ ladder ” method?
1 st – Determine your starting point.
2 nd – Count the “ jumps ” to your ending point.
3 rd – Move the decimal the same number of jumps in the same direction.
4 km = _________ m
Starting Point Ending Point
How many jumps does it take?
4.
1
__
.
2
__
.
3
__
. = 4000 m
Conversion Practice
Try these conversions using the ladder method.
1000 mg = _______ g 1 L = _______ mL 160 cm = _______ mm
14 km = _______ m 109 g = _______ kg 250 m = _______ km
Compare using <, >, or =.
56 cm 6 m 7 g 698 mg
Metric Conversion Challenge
Write the correct abbreviation for each metric unit.
1) Kilogram _____
2) Meter _____
4) Milliliter _____
5) Millimeter _____
7) Kilometer _____
8) Centimeter _____
3) Gram _____ 6) Liter _____
Try these conversions, using the ladder method.
9) Milligram _____
10) 2000 mg = _______ g 15) 5 L = _______ mL 20) 16 cm = _______ mm
11) 104 km = _______ m 16) 198 g = _______ kg 21) 2500 m = _______ km
12) 480 cm = _____ m 17) 75 mL = _____ L 22) 65 g = _____ mg
13) 5.6 kg = _____ g 18) 50 cm = _____ m 23) 6.3 cm = _____ mm
14) 8 mm = _____ cm 19) 5.6 m = _____ cm 24) 120 mg = _____ g
Compare using <, >, or =.
25) 63 cm 6 m 27) 5 g 508 mg 29) 1,500 mL 1.5 L
26) 536 cm 53.6 dm 28) 43 mg 5 g 30) 3.6 m 36 cm
Metric Prefixes
-indicates how many times a unit should be multiplied or divided by ten
Figure 15 on page 17 shows some common metric prefixes
EX 9ms = 9 s = 0.009s
1000
*Note – dividing by 1000 same as multiplying by 0.001
http://www.metricamerica.com/mediasays.htm
http://www.homeschoolmath.net/worksheets/measuring.php
More on metric prefixes
-can make a unit larger
EX 12 km = 12 x 1,000m = 12,000 m
Conversion Factors – a ratio of equivalent measurements that is used to convert a quantity expressed in one unit to another unit.
Let ’ s look together on page 18 of you text to work through an example of this
FACTOR LABEL Method
Measuring Temperature
Thermometer – thermo (heat) meter (measure)
An instrument that measures temperature, or how hot an object is.
Let ’ s look at page 21 together
Fahrenheit and Celsius and kelvin conversions
Copy the formulas found on page 20 into your notes
Practice converting temp.
K = 0 C + 273 formula
Convert
205 K = ______ 0 C
205k – 273 = -68 0 C
42 0 C = ___ K
42 0 C + 273 = 315K
More practice
0 C = (F – 32) x 5/9 AND F = 0 C x 9/5 + 32
Copy the example from the bottom of Page 878 in your text into your notes
Do the following conversions on your own for practice using the example you just copied
33.182F = _____ 0 C
14F = _____ 0 C
One more set of practice in F
65C = _____F
22C = _____F
What unit to use when?
Length Temperature
Mass Volume
Time Electric Current
Second (s) Ampere (a)
Kelvin (k) Meter (m)
Liter (l) Kilogram (kg)
Presenting Sci. Data
Organizing Data – Scientists organize data using tables and graphs.
These make it easier to spot patterns/trends to support of disprove their hypothesis
Data Tablesthe simplest way to organize data
Organizing data
Line Graphs – Useful for showing changes that occur in related variables
Manipulated variable – x-axis
Responding variable – y-axis
Slope = Rise
Run
Direct proportion –As one goes up/down the other goes up/down the same amount
Inverse proportion – As one goes up/down the other goes down/up
Thumb demonstration
Look at fig. 22
Do Data Analysis page 24
More organizing data
Bar Graphs- often used to compare a set of measurements , amounts, or changes.
Circle Graphs- a divided circle that shows how the part or share of something relates to the whole.