Carbon Chemistry
advertisement

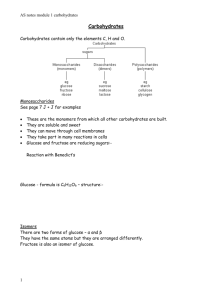
Carbon Chemistry • Carbon atoms can form single, double or triple bonds with other carbon atoms. • Carbon can form up to 4 bonds • This allows carbon atoms to form long chains, almost unlimited in length. Macromolecules • “GIANT MOLECULES” • Made up of thousands of little molecules. • Formed from a process known as polymerization, in which large molecules are produced by joining small ones together. • The small units (monomers), join together to form large units (polymers) Where Do Carbohydrates Come From? • Plants take in Carbon dioxide (CO2) and water (H2O) + heat from the sun and make glucose. • C6H12O6 Carbohydrates • As the name implies, consist of carbon, hydrogen, and oxygen. • Hydrate=(water) hydrogen and oxygen. • The basic formula for carbohydrates is CH2O, meaning that there is one carbon atom, two hydrogen atoms, and one oxygen atom as the ratio in the structure of carbohydrates • What would be the formula for a carbohydrate that has 3 carbons. • C3H6O3 Carbohydrate • Fancy way of saying sugar. • Carbohydrates are energy packed compounds, that can be broken down quickly by organisms to give them energy. • However, the energy supplied by carbohydrates does not last long, and that is why you get hungry every 4 hours. • Carbohydrates are also used for structure. Saccharides • Scientist use the word saccharides to describe sugars. • If there is only one sugar molecule it is known as a monosaccharide • If there are two it is a disaccharide • When there are a whole bunch, it is a polysaccharide. Glucose is a monosaccharide • Notice there is only one sugar molecule. • Glucose is the main fuel for all living cells. • Cells use glucose to do work. Disaccharide Maltose • Maltose is an example of a disaccharide • Notice it is two sugar molecules together. • Glucose + Glucose = Maltose The most common disaccharide is Sucrose • Sucrose is glucose + fructose and is known as common table sugar. Polysaccharide • Polysaccharides are a whole bunch or monosaccharides linked together. • An example of a polysaccharide is starch. Most of the names of carbohydrates end in -ose • Glucose-What plants make • Maltose- used in making beer (disaccharide) • Fructose – found in fruit (monosaccharide) • Sucrose- Table sugar (disaccharide) • Lactose – In milk (disaccharide) Isomers • Glucose • C6H12O6 • Fructose • C6H12O6 • Fructose sweeter then glucose because of its structure. Glucose can be fond in a ring structure or linear structure • In Water Dehydration Synthesis • Sounds technical but all it really means is taking out the water and making some thing new. • Dehydration is what happens to you when you don’t drink enough water. • Synthesis means “making some thing new” • In this case we are taking out water and connecting glucose with fructose to make sucrose (table sugar) Hydrolysis Hydro=water lysis= break apart • Hydrolysis breaks down a disaccharide molecule into its original monosaccharides. • Hydrolysis, it means that water splits a compound. • When sucrose is added to water, it splits apart into glucose and fructose. Do Too many carbohydrates make us fat! • If you eat more calories than you expend in energy, then anything can be stored as fat - protein, fat or carbohydrate. However if you do not take in any carbohydrates into your body you will use fat as fuel. Thank god for the low carb diet. Now, nobody wants to eat me because they think I make them fat. What do we do with all the sugar? • Plants store glucose in the form of polysaccharides known as starch in their roots . • Animals store glucose in the from of a polysaccharide known as glycogen in our liver and muscle cells. Cellulose • The most abundant organic molecule on earth. • Gives trees and plants structure and strength. • Most animals can not break the glucose linkage by normal means of hydrolysis. Need special enzymes. • We need cellulose (fiber) to keep our digestive tracts clean and healthy. Polysaccharides are used in the shell of crustaceans like crabs and lobsters. Chitin Carbohydrates also serve as structural elements. • The chains sticking out of the proteins in the cell membrane are polysaccharides know as cell markers. How Sweet It Is • The human tongue has four basic taste qualities. • Bitter • Salty • Sour • Sweet • We perceive taste qualities when receptors on our tongue send a message to our brain. • Sucrose taste sweet but so does equal. • Sucrose is made of sugars • Equal is made of amino acids linked together it taste sweet also, so what is going on here. Its all about how tightly the molecules fit into the receptors on the tongue. • The chemical structure of a compound determines its shape, which in turn will determine how well it will fit into a receptor. • Compounds that bind more tightly to “sweet” taste receptors send stronger “sweet” messages to the brain.