Types of Energy Foldable
advertisement

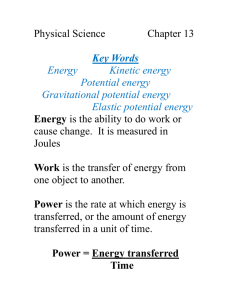
Types of Energy Foldable Definition of Energy Energy is the ability to do WORK. It is measured in the UNIT Joules (J). BUT….what is work? o Work is when force is used to move an object a distance. • SO---Energy is ability to produce a change in motion/position due to a force. Law of Conservation of Energy The Law of Conservation of Energy STATES: Energy cannot be created nor destroyed, only changed (from one form into another or transferred from one object to another). Kinetic Vs. Potential Energy • kinetic energy (energy of motion)—Moving energy • potential energy (stored energy)—Energy is building Kinetic Vs. Potential Energy Honors The formula for calculating the kinetic energy of an object is • KE = ½ mv² where: – m is the mass of the object – v is its speed of velocity and v² is the velocity squared or v times v – ½ mv² is one-half times m times v² The formula for calculating the Potential energy of an object is • PE = mgh where: – – – – – PE is the potential energy m is its mass g is the acceleration of gravity (32 ft/s² or 9.8 m/s²) mg is the weight of the object (m times g) h is the height of the object from the floor or ground Mechanical Energy Can be either kinetic energy (energy of motion) or potential energy (stored energy of position). Occurs within AN OBJECT due to its motion or due to its position Mechanical Energy Examples • • • • • Wind Water A moving car A book on a shelf Sound Chemical Energy Potential Energy (until the chemical reaction puts the atoms/molecules in motion (kinetic). Energy that is stored in the forces that hold, or bond, atoms together and is released when the atomic structures/bonds are changed. Each bond holds a specific amount of energy. Chemical Energy Examples Food: Natural gas Petroleum Propane Biomass (Biodegradable wastes that can be burnt as fuel) Thermal Energy Kinetic Energy Known as heat Occurs as a result of the movement and vibrations of the molecules and atoms of the substance (the faster they move, the hotter the substance). The increase/decrease of movement can cause substances to change phases; for example, heating water in order to produce steam. Thermal Energy Examples • • • • Geothermal energy Volcano Boiling a pot of water Melting Ice Cream Transferred through Convection, Conduction, Radiation Electrical Energy Potential energy when a circuit is open, Kinetic energy once the switch is flipped (closed) and the charges move! Occurs as electrical charges (electrons) are forced to be in motion. Electrical Energy Examples • Lightning • The movement of electrical charges through a wire (like our circuits, electrical outlets) *Anything plugged in or a switch that closes to allow flow of electricity! Electromagnetic Energy AKA Radiant AKA SOLAR Energy AKA Light Energy Kinetic energy Provides the earth’s heat (in addition to the core) and light Travels in waves that are transverse through open spaces and through a vacuum! Can be visible or invisible Electromagnetic Energy AKA Radiant AKA SOLAR Energy AKA Light Energy Examples Light X-rays Gamma rays Radio waves Ultraviolet rays Infrared rays Nuclear Energy Potential Energy Energy is stored in the nucleus of an atom, holding it together. Energy is released when: o The nuclei are combined (fusion) i.e., when hydrogen atoms combine from the power of the sun. o The nuclei are split apart (fission) i.e., when uranium atoms are split in a nuclear power plant. Nuclear Energy Examples • Stars (like our sun)— – Fusion –Nuclei Combine Nuclear Energy Examples Nuclear reactors—Fission-Nuclei are split*Nuclear Power Nuclear Energy Examples • Uranium atoms are split (fission-Nuclear Energy) which produces heat to boil water into steam (Thermal Energy), which turns turbines (Mechanical Energy) to produce Electrical Energy! • **The main difference is that with nuclear fission the heat generated from the fission heats the water instead of the water being heated by using oil, gas or coal (which is chemical energy). Additional Information • Although the examples of the six forms of energy may seem simple and clear cut, in reality energy and its various forms is complex. For instance: • Some types of energy are classified as both potential and kinetic. • Other energy forms have characteristics that overlap, falling into more than one energy type. • There are many additional names of energy types such as sound energy, motion energy, wave energy and gravitational energy. There is visible and invisible energy and energy that is renewable and non-renewable. The bottom line is….. • That there are several types of energy and those types of energy can be converted into each other. BECAUSE: • Energy cannot be created nor destroyed, only changed or converted!! • This is the Law of Conservation of Energy!