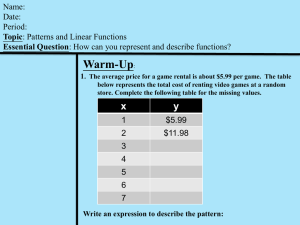
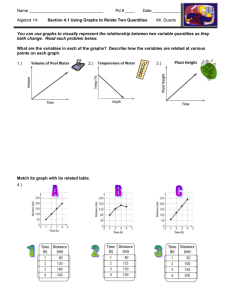
Quantities and units
advertisement

Unit 1 – Introduction to Physics QUANTITIES AND UNITS VOCABULARY Physical quantities mass force Current unit SI units International system kilogram second Basic quantities meter ampere Derived quantities Kelvin mole Candela scale periods Avogadro’s number triple point Absolute temperature scale black body QUANTITIES AND UNITS Physical quantities are things such as mass, force, and current, which can be quantified by measurement. All physical quantities have to be measured in some way and each therefore has its own unit. Units are chosen by international agreement and are called SI units abbreviated from the French (Systeme International d’Unite). QUANTITIES AND UNITS All quantities are classified as either basic quantities or derived quantities. Basic quantities are a set of quantities from which all other quantities can be defined. Each basic quantity has its basic SI unit, in terms of which any other SI can be defined. Insert basic quantity table QUANTITIES AND UNITS Basic SI Units Kilogram (kg) is the SI unit of mass. It is equal to the mass of a platinum iridium cylinder kept at Sevres, near Paris. Second (s) is the SI unit of time. It is equal to the period of a type of radiation emitted by the cesium-133 atom. QUANTITIES AND UNITS Meter (m) is the SI unit of length. It is equal to the distance light travels in a vacuum in 1/299,792,458 of a second. Ampere (A) is the SI unit of electric current. It is equal to the size of a current flowing through wires in a vacuum, that produces a force of 2 X 10-7 N every meter. QUANTITIES AND UNITS Kelvin (K) is the SI unit of temperature. It is equal to 1/273.16 of the temperature of the triple point of water on the absolute temperature scale. Mole (mol) is the SI unit of the quantity of a substance. It is equal to the amount of substance which contains 6.023 X 1023 particles. (Avogadro’s number) QUANTITIES AND UNITS Candela (cd) is the SI unit of intensity of light. It is equal to the strength of light from 1/600,000 m2 of a black body at the temperature of freezing platinum and at a pressure of 101,325 N/m2. A black body is a hypothetical object which absorbs all radiation that falls on it, and is also the best radiator (heat energy emitter). QUANTITIES AND UNITS Prefixes A given SI unit may sometimes be too large or small for convenience. Example: The meter is too large for measuring the thickness of a piece of paper. QUANTITIES AND UNITS Prefixes Standard fractions and multiples of the SI units are therefore used and written by placing a prefix before the unit. Example: 1 millimeter (mm) is equal to 1/1000 of a meter. Thickness of a sheet of paper is 0.1 mm which equals .0001 m or 1/10,000 of a meter . Insert Fractions and multiples table QUANTITIES AND UNITS Derived quantities are quantities other than basic quantities. Derived quantities have derived SI units which are defined in terms of the basic SI units or other derived units. They are determined from the defining equation for the quantity and are sometimes given special names. Insert derived quantity table