HPS_CHAPTER_2
advertisement

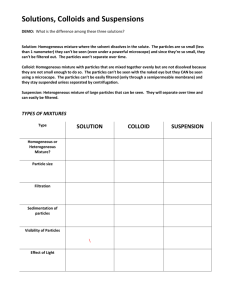
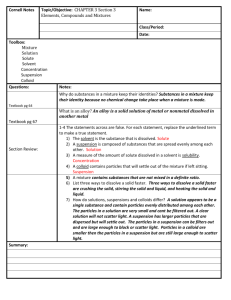
CHAPTER 2 How to classify? Devise a classification system for the following list of things: Orange Lime Plum Apple Pear Rose Violet Daisy Gold Silver Explain what criteria you used to place each item. CLASSIFYING MATTER MATTER PURE SUBSTANCE ELEMENT COMPOUND MIXTURE HOMOGENEOUS HETEROGENEOUS definitions • Pure Substance: – MATTER THAT ALWAYS HAS THE SAME COMPOSITION • Mixture: – A PHYSICAL COMBINATION OF 2 OR MORE SUBSTANCES more definitions Two categories of Pure Substances: Element – Can’t be broken down into simpler substances – Organized on Periodic Table – Each contain different types of atom – Amazing: Only about 110 different atoms make Elements some more Element • 1 or 2 letters (if 2, only first letter is capitalized) • Why? – Easier – shorter for lazy scientists – Helps scientists of all nationalities communicate AZOTE STICKSTOFF Both have symbol N Compound • Can be broken down only by chemical reaction • Examples: H2O SiO2 C6H12O6 • Proportions are FIXED – All water everywhere forever has 2 H and 1 O • Properties of Compound are DIFFERENT from the elements it’s made from Na + Cl2 NaCl Mixtures • Definition: 2 or more substances physically combined • Examples: fruit salad, salt water, steel, sand, striped cloth, maple syrup • Keep some (most) of their individual properties. • Classified by how well the substances are mixed. Heterogeneous Mixture • The parts are noticeably different from one another. • fruit salad, sand, striped cloth Homogeneous Mixture • The parts are so evenly mixed the individual parts are difficult or impossible to distinguish. • Sea water, steel, maple syrup Special Types of Mixtures 1. SOLUTION Formed when one substance DISSOLVES in another Kool-Aid, Antifreeze, Oxygen in water Dissolved particles are so small: – Do not separate over time – If filtered, no particles are trapped – Light will pass through without being scattered 2. SUSPENSION A heterogeneous mixture that will separate over time. Muddy water, OJ with pulp, Apple cider Dissolved particles are larger: – Will separate over time – If filtered, particles will be trapped – Light passing into it is scattered in all directions 3. COLLOID A mixture with larger particles than solution but smaller than suspension Fog, milk, styrofoam, blood, gelatin Medium-sized particles – Will not separate into layers – A filter traps no particles – Will scatter light in all directions CHECK YOURSELF Place the following in order from smallest to largest particle size SUSPENSION, SOLUTION, COLLOID SOLUTION SMALLEST COLLOID SUSPENSION LARGEST • Fill in the diagram MATTER PURE SUBSTANCE ELEMENT MIXTURE COMPOUND HOMOGENEOUS SOLUTION COLLOID HETEROGENEOUS SUSPENSION Physical Properties Any characteristic that can be measured or observed without changing the composition of the material. • VISCOSITY – Ability to flow…slow flowing (syrup) = viscous • CONDUCTIVITY – Ability to conduct heat and/or electricity • MALLEABILITY – Ability to bend or be hammered without breaking • HARDNESS – Measures resistance to shape change • Melting Point – Temp at which particles become free to pass each other • Boiling Point – Temp at which particles gain total freedom from one another • Density – Amount of matter per unit volume m D V Uses of Physical Properties 1. Identify a substance: List of properties is like a fingerprint 2. Choosing a substance: – Bulletproof vest? – Artificial Heart? 3. Mixture Separation – Filtration Uses differences in particle size – Distillation Uses differences in boiling point Divide this list into two groups: • • • • Burning a marshmallow • Rusting car Cutting paper • Dissolving salt in water Melting an ice cube • Baking soda + Vinegar Smashing a light bulb • • • • Dissolving salt in water Cutting paper Melting an ice cube Smashing a light bulb • Burning a marshmallow • Rusting car • Baking soda + Vinegar • • • • Dissolving salt in water Cutting paper Melting an ice cube Smashing a light bulb This list: All change the shape/form but the substance remains the same PHYSICAL CHANGES Chemical Properties Can be observed only when the substance is changing into a different substance • Burning a marshmallow • Rusting car • Baking soda + Vinegar Two Names: 1. Flammability – Ability to burn in the presence of oxygen 2. Reactivity – describes how readily a substance combines chemically with another Sodium is reactive with water Iron is not reactive with water Magnesium is reactive with acid Recognizing Chemical Change • Color Change – Copper turns green with age – Rust • Form a Gas – Baking soda + Vinegar – Magnesium + Acid • Form a Precipitate – lead nitrate + sodium iodide Practice with P/C Changes Shattering Glass Sunburn Bleaching clothes Baking cookies Folding laundry Ripping your pants Hydrogen peroxide on a cut P P P P P P P C C C C C C C