Chem 30BL-Lecture 9b..
advertisement

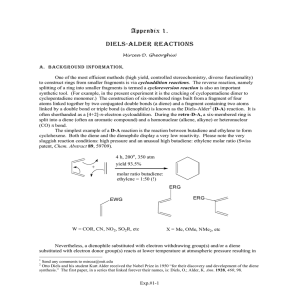
Diels-Alder Reaction II A diene and dienophile undergo a cycloaddition Prototype reaction: butadiene and ethylene [4n+2]p-addition + diene dienophile “aromatic TS” cyclo-adduct In order for the aromatic transition state to form, the diene has to be s-cis conformation (cisoid) s-cis DHf= 73.16 kJ/mol s-trans 72.54 kJ/mol + CH2=CHCOCH3 s-trans s-cis DHf= 79.12 kJ/mol N.R. 40.53 kJ/mol Substituents on the diene and the dienophile can have a significant effect on their reactivity and therefore the conditions required to carry out the reaction 200oC + O O CH3 140oC CH3 + O + O CH3 30oC CH3 Strategically placed donor and acceptor groups both seem to facilitate the reaction Explanation (simplified version) Energy LUMO LUMO LUMO LUMO LUMO HOMO HOMO LUMO HOMO HOMO HOMO O HOMO O Acceptor groups lower the orbital energies for the HUMO and the LUMO orbital because they reduce the electron-density in the p-system Donor groups raise the orbital energies for the HUMO and the LUMO orbital because they increase the electron-density in the p-system Thus, placing a donor in the diene and an acceptor in the dienophile (or vice versa inverse electron-demand) is most effective in decreasing the HUMO-LUMO gap, which directly relates to the activation energy for the reaction Diels-Alder reactions are stereoselective because they are concerted for most parts (=all bonds are broken and formed at the same time) The stereochemistry of the diene and the dienophile are retained in the cyclo-adduct O H Cis to cis + H H CH3 CH3 CH3 CH3 H O O O Trans to trans H H O CH3 + H3C O CH3 CH3 H O H O For many Diels-Alder reactions, a high degree of regioselectivity is observed favoring six-membered rings with 1,2- or 1,4-substitution R R R X X + X major X minor X + R R R X minor major Example: Reaction of 1-methoxybutadiene (R=OMe) and methyl vinyl ketone (X=COCH3) O O Resonance contributors of the diene O O Resonance contributors of the dienophile If a bicycle is formed in the reaction, an endo or an exo product can be formed in the reaction depending on the temperature Endo product R R H + R R R H R Exo product + R R R R R H R H Which product is preferentially formed highly depends on the reaction conditions Low temperature: endo product High temperature: exo product Example: Maleic anhydride and cyclopentadiene Exo approach This product is usually thermodynamically more stable O O O LU MO O O C O O H HOMO O H C Endo approach This product is formed at lower temperatures because of the secondary orbital interaction (in red) which lower the activation energy for the endo pathway O C O LU MO O O O LU MO H C O O H C C O O HOMO HOMO The activation energies for the endo and the exo pathway are different resulting different rates of reaction Y= Eact( 1) Eact( 2) Eact( 3) Eact( 4) A+B Energy Y X= H O HC C O O O CO HC H O A= (start) X Endo (Kin etic Product) Exo DHf= -293.3 kJ/mol (AM1) DHf= -300.7 kJ/mol (AM1) (T herm ody nam ic Pro duct) Reactant O B= O Reactant rxn coordinate O The endo pathway has the lower activation energy and is therefore favored at low temperatures (=kinetic control) The endo-product can be converted to the exo-product by heating (T=190 oC, 1.5 hrs) because the reaction is reversible (=thermodynamic control) At high temperatures (T=206 oC), an almost equimolar mixture of the endo and the exo-product is obtained from the reaction of dicyclopentadiene with maleic anhydride The simplest reaction would be the reaction of ethylene with itself + no lig ht at r.t. ? Experience tells us that this reaction does not take place Ethylene has only two p-electrons p LUMO p HOMO The combination of the HUMO and LUMO of ethylene leads to one bonding and one anti-bonding Energy antibonding interaction, which cancel each out other energetically + bonding XXX no reaction The next case would be the reaction of ethylene with butadiene + This reaction seems to proceed with low yields (~20 %) p anti-bonding orbitals p3 LUMO p2 HOMO Energy bonding orbitals p Butadiene has four p-electrons, which means that the two lowest p-orbitals are filled making p2 the HOMO and p3 the LUMO Either combination leads to the formation of two new bonding interactions and no anti-bonding interactions bonding bonding LUMO HOMO Cycloadduct HOMO bonding bonding LUMO The cyclo-adduct is formed in this reaction Reactions that involve [4n+2]p-electrons are allowed thermodynamically speaking (D) Reactions that involve [4n]p-electrons often require photochemically activation (hn)