Bacteria
advertisement
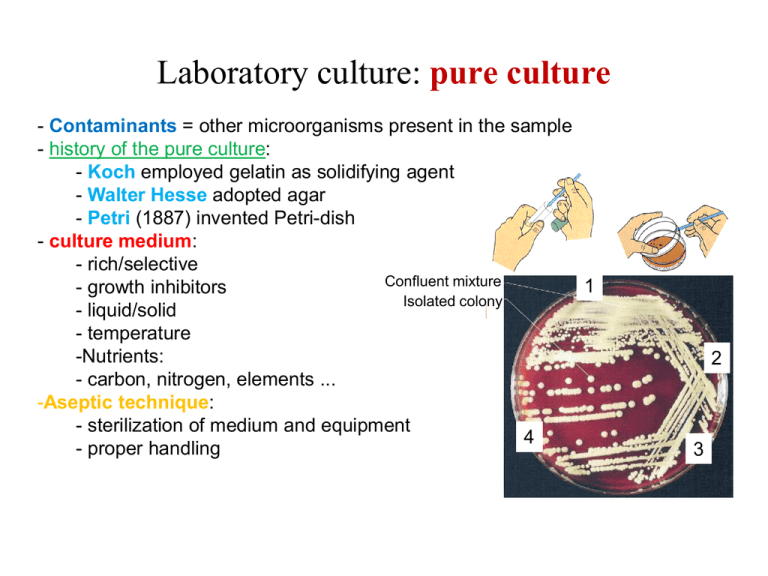
Laboratory culture: pure culture - Contaminants = other microorganisms present in the sample - history of the pure culture: - Koch employed gelatin as solidifying agent - Walter Hesse adopted agar - Petri (1887) invented Petri-dish - culture medium: - rich/selective Confluent mixture 1 - growth inhibitors Isolated colony - liquid/solid - temperature -Nutrients: - carbon, nitrogen, elements ... -Aseptic technique: - sterilization of medium and equipment 4 - proper handling 2 3 Bacterial growth: basic concepts Precursors Anabolism = biosynthesis Catabolism = reactions to recover energy (often ATP) Bacterial growth: basic concepts troph = „to feed“ (where does energy come from?) Chemolithotroph = inorganic compounds as energy source Chemoorganotroph = organic compounds as energy source Microbial nutrition Nutrients = chemical „tools“ a cell needs to grow/replicate Macronutrients = chemicals needed in large amounts Micronutrients = chemicals needed in small/trace amounts Autotrophy = CO2 can be sole C-source % of dry weight 50% 12% (sometimes non-essential) (sometimes non-essential) Microbial nutrition: Growth factors - organic compounds required by some bacteria - vitamins, amino acids, purines, pyrimidines - Streptoccus, Lactobacillus, Leuconostoc (lactic acid bacterium): complex vitamin requirements Microbial growth media - chemically defined: highly purified inorganic and organic compounds in dest. H2O - complex (undefined): digests of casein, beef, soybeans, yeast, ... Microbial growth media Media *Complex Purpose Grow most heterotrophic organisms *Defined Grow specific heterotrophs and are often mandatory for chemoautotrophs, photoautotrophs and for microbiological assays *Selective Suppress unwanted microbes, or encourage desired microbes *Differential Distinguish colonies of specific microbes from others *Enrichment stimulating Similar to selective media but designed to increase the numbers of desired microorganisms to a detectable level without stimulating the rest of the bacterial population *Reducing Growth of obligate anaerobes Bacterial growth Growth rate = Dcell number/time or Dcell mass/time 1 generation Growth = increase in # of cells (by binary fission) generation time: 10 min - days Bacterial growth: exponential growth Generation time = 30 min Bacterial growth: exponential growth Semilogarythmic plot Straight line indicates logarithmic growth Bacterial growth: calculate the generation time t g= n The generation time is the time needs the culture population to double t = time of exponential growth (in min, h) g = generation time (in min, h) n = number of generations Bacterial growth: calculate the generation time t g= n t = time of exponential growth (in min, h) g = generation time (in min, h) n = number of generations Bacterial growth: calculate the generation time t = time of exponential growth (in min, h) g = generation time (in min, h) n = number of generations t g= n Nt = N0 x 2n Nt = number of cells at a certain time point N0 = initial number of cells n = number of generations Bacterial growth: calculate the generation time t = time of exponential growth (in min, h) g = generation time (in min, h) n = number of generations t g= n Nt = N0 x Nt = number of cells at a certain time point N0 = initial number of cells n = number of generations 2n logNt = logN0 + n x log2 logNt - logN0= n x log2 n= logNt - logN0 log2 n = 3.3 x (logNt - logN0) Bacterial growth: calculate the generation time Im Erlenmeyerkolben wurde eine E. coli Kultur angesetzt. Die Kultur befindet sich in der exponentiellen Wachstumsphase. Die Geschwindigkeit des bakteriellen Wachstums wurde gemessen: 12.00 Uhr 103 Bakterien/ml 14.00 Uhr 1.6 x 104 Bakterien/ml Generationszeit = ? Bacterial growth: calculate the generation time Im Erlenmeyerkolben wurde eine E. coli Kultur angesetzt. Die Kultur befindet sich in der exponentiellen Wachstumsphase. Die Geschwindigkeit des bakteriellen Wachstums wurde gemessen: 12.00 Uhr 103 Bakterien/ml 14.00 Uhr 1.6 x 104 Bakterien/ml Generationszeit = ? n = 3.3 x (logNt - logN0) = 3.3 x (log1.6 x 104 – log103) = 3.3 x (4.2 – 3) = 4 2h t g = n = 4 = 0.5 h Bacterial growth: batch culture Batch culture: Lag phase no Lag phase: Inocculum from exponential phase grown in the same media Lag phase: Inocculum from stationary culture (depletion of essential constituents) After transfer into poorer culture media (enzymes for biosynthesis) Cells of inocculum damaged (time for repair) Batch culture: exponential phase Exponential phase = log-phase Maximum growth rates „midexponential“: bacteria often used for functional studies Batch culture: stationary phase Bacterial growth is limited: - essential nutrient used up - build up of toxic metabolic products in media Stationary phase: - no net increase in cell number - „cryptic growth“ - energy metabolism, some biosynthesis continues - specific expression of „survival“ genes Batch culture: death phase Bacterial cell death: - sometimes associated with cell lysis - 2 Theories: - „programmed“: induction of viable but non-culturable - gradual deterioration: - oxidative stress: oxidation of essential molecules - accumulation of damage - finaly less cells viable Measurement of microbial growth A. Weight of cell mass B. number of cells: - Total cell count - Viable count - Dilutions - turbidimetric total cell count A. Sample dried on slide B. Counting chamber: Limitations: - dead/live not distinguished - small cells difficult to see - precision low - phase contrast microscope - not useful for < 106/ml viable cell count synonymous: plate count, colony count 1 viable cell 1 colony cfu = colony forming unit Advantage: high sensitivity; selective media Optimal: 30 – 300 colonies per plate ( plate appropriate dilutions) spread plate method: pour plate method: Bacteria must withstand 45°C briefly dilutions Example: 3 h culture of E. coli in L-broth How do I determine the actual number? Turbidimetric measurements Relationship between OD and cfu/ml must be established experimentally Exponential culture of E. coli in L-broth: 1 OD = ca. 2-3 x 109 cfu/ml Turbidimetric measurements Limits of sensitivity at high bacterial density „rescattering“ more light reaches detector Klett units Klett units Two typical growth curves in batch culture 1 Klett unit = OD/0.002 Continuous culture: the chemostat steady state = cell number, nutrient status remain constant Control: 1. Concentration of a limiting nutrient 2. Dilution rate 3. Temperature Independent control of: - Cell density - Growth rate Continuous culture: the chemostat 1. Concentration of a limiting nutrient Results from a batch culture Continuous culture: the chemostat 2. Dilution rate Factors affecting microbial growth • • • • • Nutrients Temperature pH Oxygen Water availability Factors affecting microbial growth: Temperature 3 cardinal temperatures: Usually ca. 30°C Factors affecting microbial growth: Temperature Arrhenius equation: Maximum temperature Thermal protein inactivation: - Covalent/ionic interactions weaker at high temperatures. - Thermal denaturation (covalent or non-covalent) - heat-induced covalent mod.: deamidation of Gln and Asn - Thermal denaturation: reversible or irreversible. Genetics: - Missense mutations: reduced thermal stability (Temp.-sens. mutants) - Heat shock response: proteases, chaperonins (i.e. DnaK ~ Hsp70) Factors affecting microbial growth: Temperature Minimal temperature: Proteins: - Greater a-helix content - more polar amino acids - less hydrophobic amino acids Membranes: - temperature dependent phase transition Thermotropic Gel: Hexagonal arranged Membrane proteins inactive (mobility/insertion) - homoviscous adaptation Tm „Fluid mosaic“ Protein function normal Growth at low Temperatures: „Homoviscous adaptation“ Homoviscous adaptation = adjustment of membrane fluidity - lowered Tm - More cis-double bonds - Reduced hydrophobic interactions - high Tm - Few cis double bonds - optimal hydrophobic interactions - mesophiles - thermophiles Fatty acid composition of plasma membrane as % total fatty acids E. coli grown at: 10°C 43°C C16 saturated (palmitic) 18 % 48 % C16 cis-9-unsat. (palmitoleic) 26 % 10 % C18 cis-11-unsat. (cis-vaccinic) 38 % 12 % „Temperature classes“ of organisms Psychrophilic vs. Psychrotolerant Sierra Nevada Psychrophiles Maximum: <20°C Optimum: <15°C Minimum: <0°C Habitats: - deep sea - glaciers red spores Chlamydomonas nivalis The snow algae Psychrotolerant Maximum: >20°C Optimum: 20-40°C Minimum: <0-4°C Habitats: much more abundant than psychrophiles - soil in temperate climate - foods - grow slowly even in fridge! Limit: Freezing - Inhibits bacterial growth - freezing: often liquid pockets - many bacteria survive - cryoprotectants (DMSO, glycerol) Growth at high temperatures Thermophilic: optimum > 45°C Soil in sun often 50°C Fermentation: 60-65°C <65°C Hyperthermophilic: optimum > 80°C Only in few areas: Hot springs: 100°C Steam vents 150-500°C Deep sea hydrothermal vents Growth at high temperatures Molecular adaptations in thermophilic bacteria Proteins - Protein sequence very similar to mesophils - 1/few aa substitutions sufficient - more salt bridges - densely packed hydrophobic cores lipids - more saturated fatty acids - hyperthermophilic Archaea: C40 lipid monolyer DNA - sometimes GC-rich - potassium cyclic 2,3-diphosphoglycerate: K+ protects from depurination - reverse DNA gyrase (increases Tm by „overwinding“) - archaeal histones (increase Tm) Bacterial growth: pH (extremes: pH 4.6- 9.4) Most natural habitats Growth at low pH Fungi: - often more acid tolerant than bacteria (opt. pH5) Obligate acidophilic bacteria: Thiobacillus ferrooxidans Obligate acidophilic Archaea: Sulfolobus Thermoplasma Most critical: cytoplasmic membrane Dissolves at more neutral pH Bacterial growth: high pH - Few alkaliphiles (pH10-11) - Bacteria: Bacillus spp. - Archaea - often also halophilic - Sometimes: H+ gradient replaced by Na+ gradient (motility, energy) - industrial applications (especially „exoenzymes“): -Proteases/lipases for detergents (Bacillus licheniformis) -pH optima of these enzymes: 9-10 Buffers in bacterial culture media pH range buffer system 1.1 - 3.5 2.2 - 4.0 3.6 - 5.6 5.0 - 8.0 5.0 - 6.6 7.2 - 9.0 8.5 - 12.9 9.2 - 10.7 10.9 - 12.0 glycine/HCl KH-phtalate/HCl Na-acetate/acetic acid KH2PO4/Na2HPO4 Na-citrate/NaOH TRIS/HCl glycine/NaOH Na2CO3/NaHCO3 Na2HPO4/NaOH Küster Thiel, Rechentafeln für die chemische Analytik, 1982, Walter de Gruyter Sambrook et al., 1989, Molecular cloning, 2nd ed. Bacterial growth: Osmosis Water acitvity p aw = p o aw: rel. Water activity p: vapor pressure of a solution p0: vapor pressure of water Osmotic pressure p= nxRxT V p: osmotic pressure n: number of dissolved particles R: universal gas constant T: temperature V: volume of the solution Semipermeable membrane high aw low aw low p high p Bacterial growth: Osmosis Soil: water activity = 0.9 – 1.0 In general: bacteria normally have higher osmotic pressure than environment = „positive water balance“ Osmophiles: - grow in presence of high sugar concentration Xerophiles - grow in „dehydrated“ environments Bacterial growth: Halophiles Halophiles: - requirement for Na+ - grow optimally in media with low water activity - Mild: 1-6 % NaCl most other organisms - Moderate: 6-15 % NaCl would be dehydrated - extreme: 15 – 30% NaCl Bacterial growth at low aw: compatible solutes Strategy: increase internal solute concentration a. Pump inorganic ions b. Synthesize organic solutes Solute must be „compatible“ with cellular processes Bacterial growth: Oxygen O2 as electron sink for catabolism toxicity of Oxygen species Aerobes: growth at 21% oxygen Microaerophiles: growth at low oxygen concentration Facultative aerobes: can grow in presence and absence of oxygen Anaerobes: lack respiratory system Aerotolerant anaerobes Obligate anaerobes: cannot tolerate oxygen (lack of detoxification) Bacterial growth: toxic forms of Oxygen triplet oxygen: ground state singlet oxygen: reactive inactivated by carotenoids produced by light, biochemically Bacterial growth: Oxygen detoxification Catalase assay Bacterial growth: Anaerobes air air air air air obl. anaerobe fac. aerobe microaerophile aerotolerant anaerobe - Closed vessels - reducing agents (i.e. thioglycolate broth) - anaerobic jar (H2-generation + Pd catalyst) - glove box (oxygen free gas) obl. aerobe Methods to exclude/reduce oxygen:








