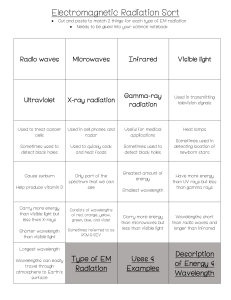
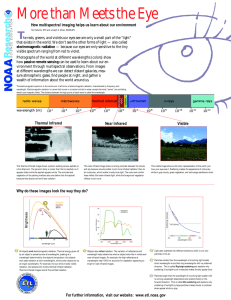
Energy and Light
advertisement

Energy and Light – Honors Chemistry Values and equations: E= hν c= λν λ = h/mν ν= [RH /h][1/ni2 – 1/nf2] Name: ___________________ RH = 2.18 x 10-18 J h= 6.626 x 10-34 J·s Show ALL work for the following problems. 1. The rays from the sun that cause tanning and burning are in the UV portion of the electromagnetic spectrum. UV – A radiation has wavelengths in the range of 320 – 380 nm, while UV – B rays have wavelengths in the 290 – 320 nm. (a) Calculate the frequency of light with a wavelength of 320. nm? (b) Calculate the energy of a photon with the frequency calculated in (a). (c) Calculate the energy of a mole of photons with a wavelength of 320. nm. (d) Which photons have more energy UV – A or UV – B? (e) UV – B radiation from the sun is considered a greater cause of sunburn in humans than is UV – A. Use the information in the problem to explain why. 2. The distance between the Mars and Earth when they’re both on the same side of the sun is approximately 5.46 x 107 km. a. How long would it take a radio wave with a frequency of 7.25 x 105 Hz to travel from Mars to Earth? b. How long would it take gamma radiation with a wavelength of 0.80 pm to reach Earth from Mars? 3. Cobalt – 60 is an artificial radioisotope that is produced in a nuclear reactor for use as a gamma-ray source in the treatment of certain types of cancer. If the wavelength of the gamma radiation from a cobalt – 60 source is 1.00x 10-3 nm, calculate the energy of a photon of this radiation? 4. A laser emits light with a frequency of 4.69 x 1014 Hz. What is the energy of one quantum of this energy? The laser emits energy in pulses of short duration. If the laser emits 1.3 x 10-2 J of energy during a pulse, how many quanta of energy are emitted during a pulse? 5. Use the de Broglie relationship to determine the wavelengths a lithium atom moving at 2.5 x 105 m/s. (1 amu = 1.66053886 × 10-24 grams) 6. For each of the following electronic transitions, is energy emitted or absorbed? a. From n = 4 to n = 2 b. From an orbit of a radius 2.12 Å to one of a radius of 8.48 Å. c. From n = 6 to n = 9 7. Complete the chart below and SHOW ALL WORK. ni 5 nf 4 2 E 6 7.57 x 10-20 J 95.0 nm Absorbed or emitted? λ (d) Do any of these transitions emit or absorb light in the visible part of the spectrum? If so, which ones?