Chapter 7/8
advertisement
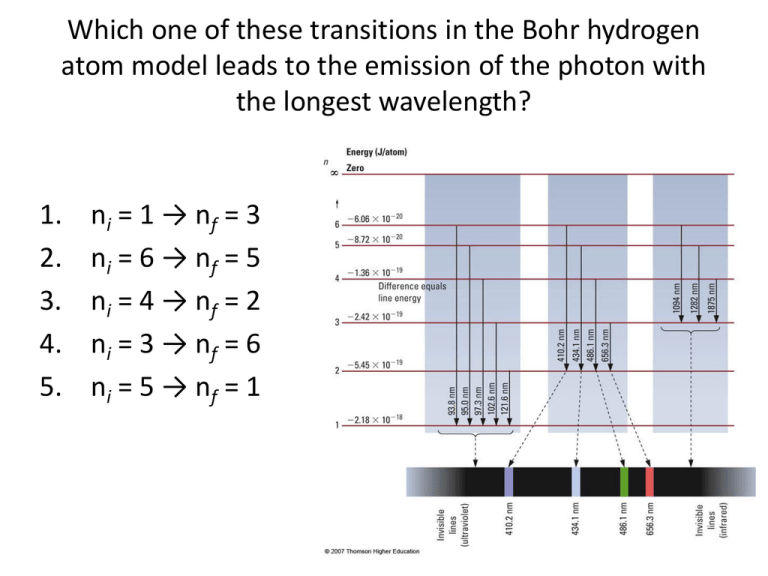
Which one of these transitions in the Bohr hydrogen atom model leads to the emission of the photon with the longest wavelength? 1. 2. 3. 4. 5. ni = 1 → nf = 3 ni = 6 → nf = 5 ni = 4 → nf = 2 ni = 3 → nf = 6 ni = 5 → nf = 1 Dental lasers are based on neodymium yttrium aluminum garnet (Nd-YAG) crystal, with an emission wavelength of 1066 nm. What is the frequency of this laser? 1. 2. 3. 4. 2.812 × 1014 Hz 2.812 × 105 Hz 3.195 × 102 Hz 3.196 × 1018 Hz The nitrogen laser emits light at 337.1 nm. What is the energy of this light? 1. 2. 3. 4. 6.696 × 10-32 J 2.118 × 10-31 J 5.893 × 10-20 J 5.893 × 10-19 J Which of the following is a list of allowed quantum numbers for an electron? 1. n = 1, l = 1, ml = 0, ms =½ 2. n = 2, l = 0, ml = 1, ms =½ 3. n = 3, l = 0, ml = 0, ms =½ 4. n = 4, l = 3, ml = 4, ms = -½ What is the correct electron configuration for sulfur? 1. 2. 3. 4. 1s22s22p63s13p5 1s22s22p63s23p4 1s22s22p73s23p2 3s23p4 What is the correct condensed electronic configuration for Fe3+? 1. 2. 3. 4. [Ar]4s23d6 [Ar]4s23d3 [Ar]3d5 [Ar]4s23d9 Which is the largest atom below? 1. 2. 3. 4. O N Al Sr Key 1. 2 2. 1 3. 4 4. 3 5. 2 6. 3 7. 4