Notes #3
advertisement
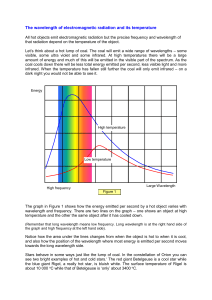
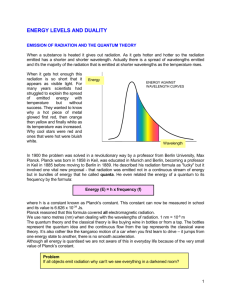
Frequency Wavelength Energy Radio: Your radio captures radio waves emitted by radio stations, bringing your favorite tunes. Radio waves are also emitted by stars and gases in space. Microwave: Microwave radiation will cook your popcorn in just a few minutes, but is also used by astronomers to learn about the structure of nearby galaxies. Infrared: Night vision goggles pick up the infrared light emitted by our skin and objects with heat. In space, infrared light helps us map the dust between stars. Visible: Our eyes detect visible light. Fireflies, light bulbs, and stars all emit visible light. Ultraviolet: Ultraviolet radiation is emitted by the Sun and are the reason skin tans and burns. "Hot" objects in space emit UV radiation as well. X-ray: A dentist uses X-rays to image your teeth, and airport security uses them to see through your bag. Hot gases in the Universe also emit X-rays. Gamma ray: Doctors use gamma-ray imaging to see inside your body. The biggest gamma-ray generator of all is the Universe. ROYGB(I?)V Review your units! Wavelength (lambda): Frequency (nu): meters (m) Hertz (Hz) or oscillations per second Faster than anything else on earth… Speed of light (c) = 3.00 x 108 meters per second (m/s) Use this number for your calculations. 670,616,629 miles per hour 186,282 miles per second 983,571,056 feet per second Wavelength and Frequency are INVERSELY PROPORTIONAL Calculate the wavelength of yellow light emitted by the sodium lamp shown above if the frequency of radiation is 5.10 x 1014 Hz (s-1). What is the frequency of radiation with a wavelength of 5.00 x 10-8 m? In what region of the electromagnetic spectrum is this radiation? h= planck’s constant= 6.626 x 10-34 joules·second (J·s) E = energy (joules) (J) v= frequency = oscillations per second (osc./s) What is the energy of a photon of microwave radiation with a frequency of 3.20 x 1011 s-1 (or Hz) ? When atoms absorb energy, electrons move into higher energy levels. These electrons then lose energy by emitting light when they return to lower energy levels. This happens by passing an electric current through a gas. 2.0 x 10-7 m to 8.0 x 10-7 m. They are giving you nanometers. They just converted the numbers . Each element has a unique emission spectrum. Like a fingerprint. Because each element has a unique emission spectrum, we can determine the composition of the universe… The atomic spectra of starts for example. They are hot glowing bodies of gases! Spectroscopist: finds the chemical contents of unknown materials in chemical research. Chemical research Police Investigations: Forensics Studies of the Universe Environmental Scientist ETC. You decide… How much of THAT is in that? Unit 1 Test : Notes #1-3 and Article Materials Thursday August 28th Lab Report 1: Spectroscopy Inquiry We will begin process on September 2. Due date to be announced.