Acid Base Review
advertisement

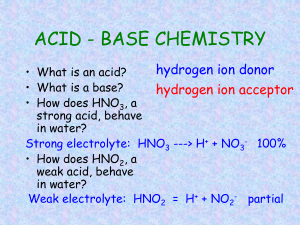
Acid/Base Quiz Review 1. Define the following terms. a. Arrhenius acid b. Arrhenius base c. Brønsted-Lowry acid d. Brønsted-Lowry base e. Conjugate acid f. Conjugate base g. Amphoteric h. pH 2. Identify the Brønsted-Lowry acid, Brønsted-Lowry base, conjugate acid, and conjugate base in the reactions below: a. HSO41- + NH3 SO42- + NH41+ b. H2O + NO31- OH- + HNO3 c. H2O + HCO31- H3O1+ + CO32- 3. Label the beaker in the picture that represents a strong acid. Label the beaker that represents a weak acid. a. What makes an acid or base strong? b. What makes an acid or base weak? c. List 7 strong acids. d. List 8 strong bases. 4. List 4 properties of an acid. List 4 properties of a base. 5. Use the pH equations below to answer these questions. pH = -log[H+] pOH = -log[OH-] pH + pOH = 14.00 [H+] = 10-pH [OH-] = 10-pOH [H+] × [OH-] = 1.00 × 10-14 M2 a. Find the pOH of a solution of HNO3 with a pH of 5.45. b. Calculate the pH of a solution of HCl with a concentration of 6.56 × 10-7 M. c. Determine the pOH of a solution of HNO3 with a [OH-] of 7.67 × 10-11 M. d. Find the pH of a solution of Ca(OH)2 with a concentration of 8.78 × 10-6 M. e. Calculate the [H+] of an HClO3 solution with a pH of 2.32. f. Calculate the [OH-] of a solution of HI with a [H+] of 9.89 × 10-4 M. g. Calculate the [H+] of a solution of Sr(OH)2 with a pOH of 3.43. 6. Use the neutralization equation to answer these questions. MAVA = MBVB a. What is the concentration of .900L HCl needed to neutralize 1.21 L of .0750 M LiOH? b. What is the volume, in L, of 1.40 × 10-4 M HClO3 needed to neutralize 125 mL of 1.10 × 10-4 M Ca(OH)2?















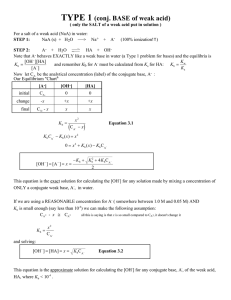



![1 K a = [H + ][CN](http://s2.studylib.net/store/data/009996856_1-2c5c638b63312695200775bae392f36d-300x300.png)