Document
advertisement

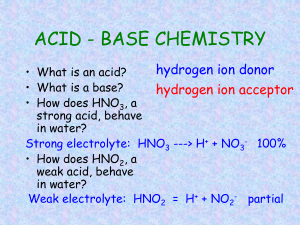
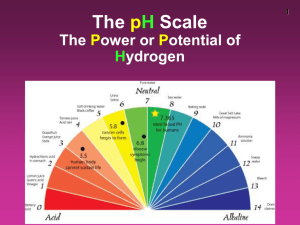
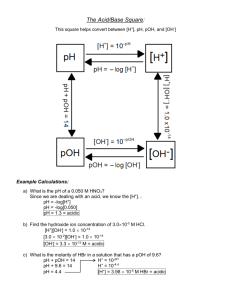
pH Water Water is in equilibrium with its ions H2O(l) H+(aq) + OH-(aq) Kw = [H+][OH-] Kw = 1.0 x 10-14 at 25°C In neutral solutions [H+]=[OH-]= 1x10-7 If [H+]>[OH-], solution is acidic If [H+]<[OH-], solution is basic Example Find the [H+] if a) [OH-]= .010M b) [OH-]= 2.0 x 10-9 M pH Very small numbers can be conveniently expressed on a log scale pH = -log [H+] pH = 7, neutral pH < 7, acidic pH > 7, basic Example Calculate the pH of lemon juice with an [H+]= 3.8 x 10-4M Calculate the pH of window cleaner with an [H+]= 5.3 x 10-9M What is the [H+] of apple juice with a pH of 3.76. pOH pOH = -log [OH-] pOH = 7, neutral pOH < 7, basic pOH > 7, acidic pH + pOH = 14.00 Indicators Used to estimate pH Colored substance that exists in either acid or base form Acid and base form are different colors By knowing the pH where it changes color, we can determine its relative pH Strong Acids and Bases Strong acids: HCl, HBr, HI, HNO3, HClO4, H2SO4 (only the 1st H) Strong bases: hydroxides of group I and II, except Be .10M HNO3 .10M H+ .75M H2SO4 .75M H+ .50M NaOH .50M OH.75M Ca(OH)2 1.5M OH- Example What is the pH of a solution of a) .010M HCl b) .010M Ca(OH)2














![pH = -log [H 3 O + ] pOH](http://s3.studylib.net/store/data/009623797_1-fb12d55980dd0a8b24e53140fd1c2f0b-300x300.png)


![1 K a = [H + ][CN](http://s2.studylib.net/store/data/009996856_1-2c5c638b63312695200775bae392f36d-300x300.png)