RadioactiveDecay-ppt
advertisement

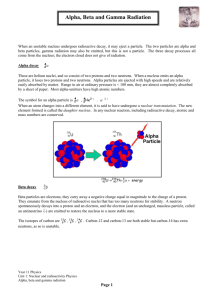
Nuclear Chemistry “Bravo” Test 1954 – 15,000 kilotons Radioactivity and Nuclear Energy Objective: To learn the types of radioactive decay Objective: To learn to write nuclear equations that describes radioactive decay Objective: To learn how one element may be changed into another by particle bombardment What makes an atom radioactive? Radioactivity: the spontaneous decomposition of a nucleus to form another nucleus and produce one or more particles. -the neutrons act as glue holding the nucleus together -the heavier the atom, the more likely it is to be radioactive -if the number of protons in the nucleus exceeds 83, then the nuclide is radioactive Types of Radioactive Decay alpha production (a, He): helium nucleus U He 238 92 4 2 234 90 Th beta production (b, e): Th 234 90 234 91 Pa e 0 1 gamma ray production (g): U He 238 92 4 2 Th 2 g 234 90 0 0 Specifying Isotopes A X Z X = the symbol of the element A = mass number (protons + neutrons) Z = the atomic number (number of protons) 5 Nuclear Symbols Mass number, A (p+ + no) 235 92 U Atomic number, Z (number of p+) Element symbol Key to Understanding Nuclear Reactions In nuclear reactions, both the atomic number Z and the mass number A must be conserved Balancing Nuclear Equations 222 226 = 4 + ____ 226 88 Ra a 4 2 222 86 Rn 88 = 2 + ___ 86 Atomic number 86 is radon, Rn Alpha Decay Alpha production (a): an alpha particle is a helium nucleus 4 2 He or a 2 U He 238 92 4 2 U a 238 92 4 2 4 2 2 234 90 Th 234 90 Th Alpha decay is limited to heavy, radioactive nuclei Alpha (α) Decay P+N P E1 P+N -4 P-2 E2 + 4 He 2 an alpha particle (helium nucleus) is produced Alpha Radiation Limited to VERY large nucleii. Example of Alpha Decay 222 88 Ra 218 86 4 Rn + 2 He Radium 222 decays by α particle production to Radon 218 Beta Decay Beta production (b): A beta particle is an electron ejected from the nucleus 0 1 Th 234 91 Th 234 91 234 90 234 90 e or 0 1 b Pa e 0 1 Pa b 0 1 Beta emission converts a neutron to a proton Beta (β) Decay P+N P E1 P+N P+1 E2 + e -1 0 Beta emission converts a neutron to a proton Beta Radiation Converts a neutron into a proton. Example of Beta Decay Notice the mass of the beta particle is zero; it is so small that is must be neglected. 14 6 C 14 7 N + -1e 0 Example of Beta Decay 234 90 Th 234 91 Pa + -1e 0 Thorium 234 decays by β particle production to Protactinium 234 (notice: no change in mass number A, and an increase of 1 in atomic number Z) Gamma Ray Production Gamma ray production (g): U He 238 92 4 2 Th 2 g 234 90 0 0 Gamma rays are high energy photons produced in association with other forms of decay. Gamma rays are massless and do not, by themselves, change the nucleus Gamma Ray Production Gamma ray production (g): U He 238 92 4 2 Gamma rays are high energy photons produced in association with other forms of decay. Gamma rays are massless and do not, by themselves, change the nucleus Th 2 g 234 90 0 0 Positron Production Positron emission: Positrons are the antiparticle of the electron 22 11 0 1 e Na e Ne 0 1 22 10 Positron emission converts a proton to a neutron Positron Production P+N P E1 P+N P-1 E2 + e 1 0 Positron emission converts a proton to a neutron Electron Capture Electron capture: (inner-orbital electron is captured by the nucleus) Hg e 201 80 0 1 Au g 201 79 0 0 Electron capture converts a proton to a neutron Alpha Particle Emission 4 Symbol 2 Mass He 2 a 4 or 2 2 Heavy How it changes the nucleus Decreases the mass number by 4 Decreases the atomic number by 2 Beta Particle Emission 0 1 e 0 or 1 b Light Converts a neutron into a proton Increases atomic number by 1 Gamma Ray Emission 0 0 g No Mass No change to the nucleus Penetration Low Medium High Protection provided by… Skin Paper, clothing Lead Danger Low Medium High Types of Radiation Nuclear Stability Decay will occur in such a way as to return a nucleus to the band (line) of stability. The most stable nuclide is Iron-56 If Z > 83, the nuclide is radioactive A Decay Series A radioactive nucleus reaches a stable state by a series of steps Graphic – Wikimedia Commons User Tosaka Alpha Particle Emission 4 Symbol 2 Mass He 2 a 4 or 2 2 Heavy How it changes the nucleus Decreases the mass number by 4 Decreases the atomic number by 2 Beta Particle Emission 0 1 e 0 or 1 b Light Converts a neutron into a proton Increases atomic number by 1 Gamma Ray Emission 0 0 g No Mass No change to the nucleus Penetration Low Medium High Protection provided by… Skin Paper, clothing Lead Danger Low Medium High