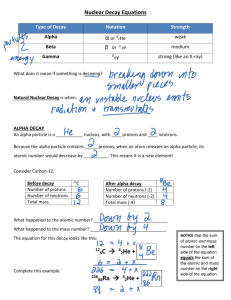
NAME NUCLEAR DECAY EQUATIONS
advertisement
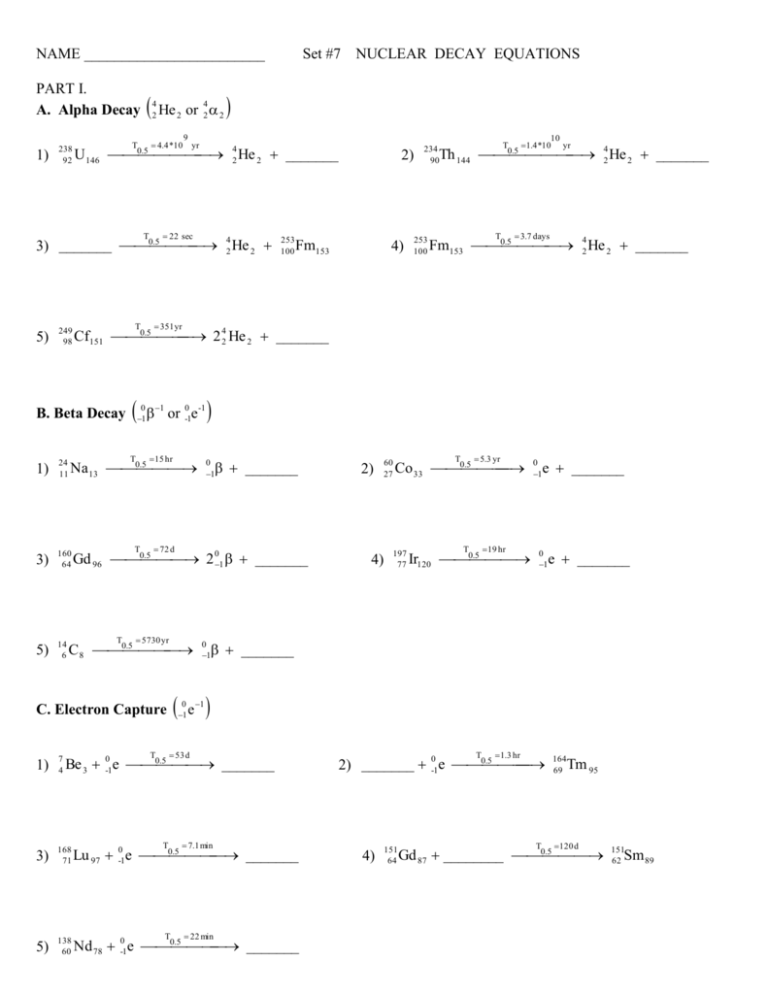
NAME ________________________ PART I. A. Alpha Decay 1) 238 92 He 4 2 or 42 2 2 4.4 *10 T 9 yr .5 U 146 0 42 He 2 _______ 22 sec T 249 98 T 0 1 1 or 0-1e-1 15 hr T 3) 160 64 5) 14 6 Fm153 4) 253 100 10 1.4 *10 yr T .5 Th 144 0 42 He 2 _______ 234 90 T 3.7 days .5 Fm153 0 42 He 2 _______ 351 yr .5 Na 13 0 24 11 253 100 2) .5 Cf151 0 2 42 He 2 _______ B. Beta Decay 1) NUCLEAR DECAY EQUATIONS .5 42 He 2 3) _______ 0 5) Set #7 T _______ 0 1 72 d .5 Gd 96 0 2 01 _______ T 5730 yr .5 C 8 0 C. Electron Capture 4) 5.3 yr T .5 Co 33 0 197 77 19 hr T .5 Ir120 0 0 1 e _______ 0 1 e _______ _______ 53 d T 60 27 0 1 0 1 1 e 2) .5 Be 3 0-1e 0 _______ 1) 7 4 3) 168 71 .5 Lu 97 0-1e 0 _______ 5) 138 60 .5 Nd 78 0-1e 0 _______ T 7.1 min T 22 min T 1.3 hr 0 .5 2) _______ -1e 0 4) 151 64 T 164 69 Tm 95 120 d .5 Gd 87 ________ 0 151 62 Sm 89 PART II 1. Write the symbol for: a. an alpha particle (2 different ways) b. a beta particle (2 different ways) c. gamma ray 2. Platinum-177 undergoes alpha decay. What will be formed? 3. Bismuth-201 is unstable and emits an alpha particle. Write an equation that shows this. 4. What would form if Sn-127 underwent beta decay? 5. Write an equation showing the beta decay of Mn-57. 6. Iron-55 has a half-life of 6.0 min. How much of a 3.88 mg sample would be left after 24 min? 7. Nitrogen-17 has a half-life of 4.16 s. If a sample has an activity of 0.226 c at some time, how long must it sit to have an activity of 0.0565 c?