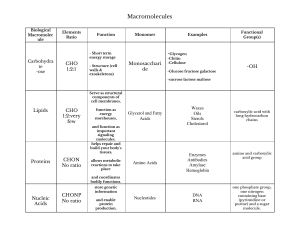
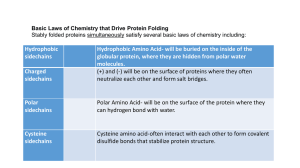
Answers
advertisement
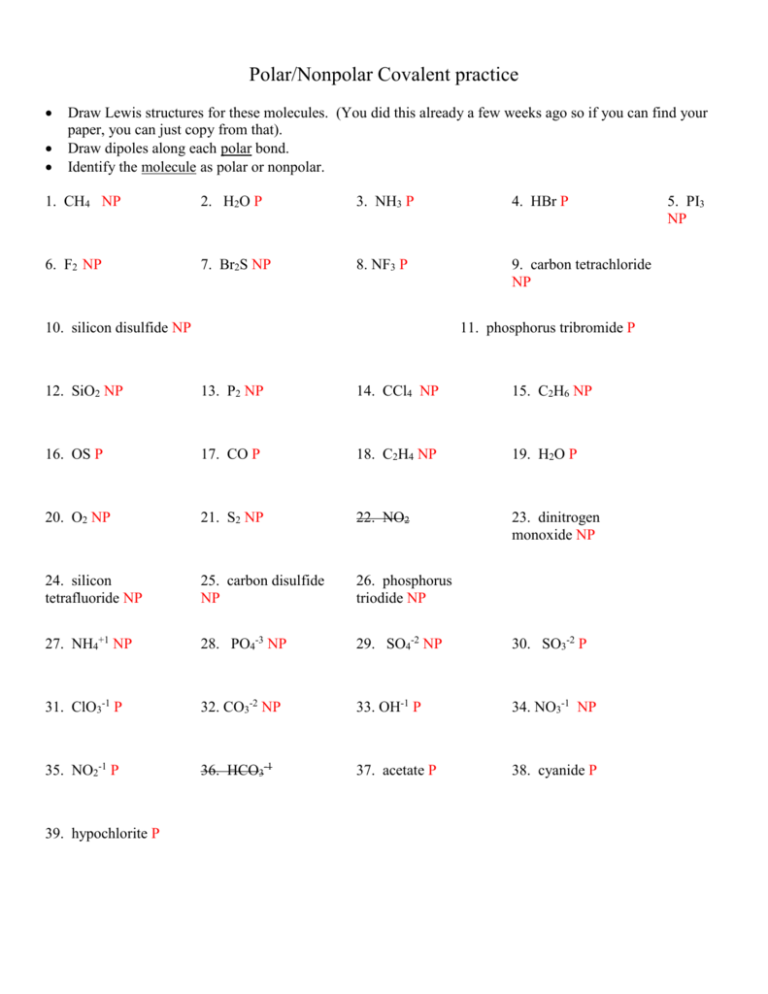
Polar/Nonpolar Covalent practice Draw Lewis structures for these molecules. (You did this already a few weeks ago so if you can find your paper, you can just copy from that). Draw dipoles along each polar bond. Identify the molecule as polar or nonpolar. 1. CH4 NP 2. H2O P 3. NH3 P 4. HBr P 6. F2 NP 7. Br2S NP 8. NF3 P 9. carbon tetrachloride NP 10. silicon disulfide NP 11. phosphorus tribromide P 12. SiO2 NP 13. P2 NP 14. CCl4 NP 15. C2H6 NP 16. OS P 17. CO P 18. C2H4 NP 19. H2O P 20. O2 NP 21. S2 NP 22. NO2 23. dinitrogen monoxide NP 24. silicon tetrafluoride NP 25. carbon disulfide NP 26. phosphorus triodide NP 27. NH4+1 NP 28. PO4-3 NP 29. SO4-2 NP 30. SO3-2 P 31. ClO3-1 P 32. CO3-2 NP 33. OH-1 P 34. NO3-1 NP 35. NO2-1 P 36. HCO3-1 37. acetate P 38. cyanide P 39. hypochlorite P 5. PI3 NP