Isotopes of Pennies Assessment: Atomic Mass Calculations
advertisement

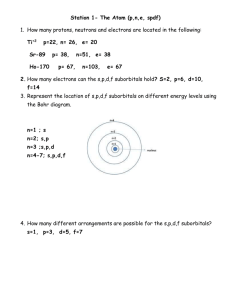
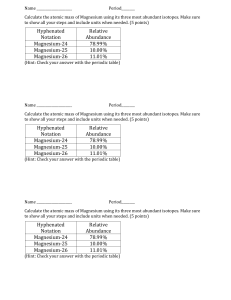
Isotopes of Pennies Assessment Sheet Problems to apply what you have learned in the Isotopes of Pennies Lab Activity by answering the questions on this sheet. 1. Calculate the average mass of potassium if the abundance and atomic masses making up its naturally occurring samples are: Potassium-39 93.12% 38.964 Potassium-41 6.88% 40.962 2. Calculate the average mass of magnesium if the abundance and atomic masses making up its naturally occurring samples are: Magnesium –24 78.70% 23.985 Magnesium –25 10.13% 24.968 Magnesium –26 11.17% 25.983 3. The atomic mass of copper is 63.540 amu. It is composed of two isotopes, Cu-63 and Cu65, with atomic masses of 62.930 and 64.928 respectively. What is the relative abundance (%) of these isotopes in naturally occurring samples of copper? 4. What is the difference between mass number and atomic mass? 5. Write a short summary explaining how this lab illustrates the concepts that you tried to explain at the beginning of the lab. Have your explanations changed? If so, explain how. Example 1: Calculate the average atomic mass of Carbon; percent abundance mass number 12 13 exact weight 12.000000 13.003355 98.90 1.10 To calculate the average atomic weight, each exact atomic weight is multiplied by its percent abundance (expressed as a decimal). Then, add the results together and round off to an appropriate number of significant figures. This is the solution for carbon: (12.000000) (0.9890) + (13.003355) (0.0110) = 12.011 amu Example 2: Copper is made up of two isotopes, Cu-63 (62.9296 amu) and Cu-65 (64.9278 amu). Given copper's atomic weight of 63.546, what is the percent abundance of each isotope? 1) Write the following equation: (62.9296) (x) + (64.9278) (1 - x) = 63.546 Once again, notice that 'x' and 'one minus x' add up to one. 2) Solve for x: x = 0.6915 (the decimal abundance for Cu-63) Note that this calculation technique works only with two isotopes. If you have three or more, there are too many variables and not enough equations. I hope it's obvious why you wouldn't do this with an element that has only one stable isotope! By the way, the 'trick' works because the other equation required is: x+y=1 We simply went right to y = 1 - x and substitued it immediately without ever writing down the second equation required.