What is Mass Spectrometry? Mass spectrometry is a procedure use
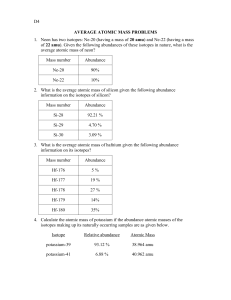
advertisement

What is Mass Spectrometry? Mass spectrometry is a procedure use to detect the isotopes that make up an atom, determine which of those isotopes are the most abundant, and this data can be used to determine the atomic mass of the element. First as sample of an element is vaporized, and then ionized to a positive ion of the element. This means that this plasma state of the element is sent through the mass spec, and guided through electrically charged plates. Once through, electromagnets are calibrated so that each isotope that is deflected by the magnets, those that weigh less are deflected more and those that are heavier are deflected less, can be detected on the ion detector. These detected particles allow for the construction of mass spec data or output, such as the graph above. This graph can then be used to determine the weighted average of all of the elements naturally occurring isotopes, also known as the elements atomic mass. Here’s how: Multiply the mass of each isotope by the percent abundance out of the total percentage (indicated by % intensity) as a decimal, and add the values together. Example from the spectrum above: For Cl-35: (35.0 x (1.00/1.37)) = 25.5 For Cl-37: (37.0 x (0.370/1.37)) = 9.99 Now add these two values up, and you should end up with the atomic mass for chlorine found on your reference table. 35.5 u (previously known as amu) is the experimental atomic mass of chlorine. If there is an extremely small margin of error, this can be due to not identifying the exact abundance from the table above, or due to significant figures.