Ch. 4 Review
advertisement
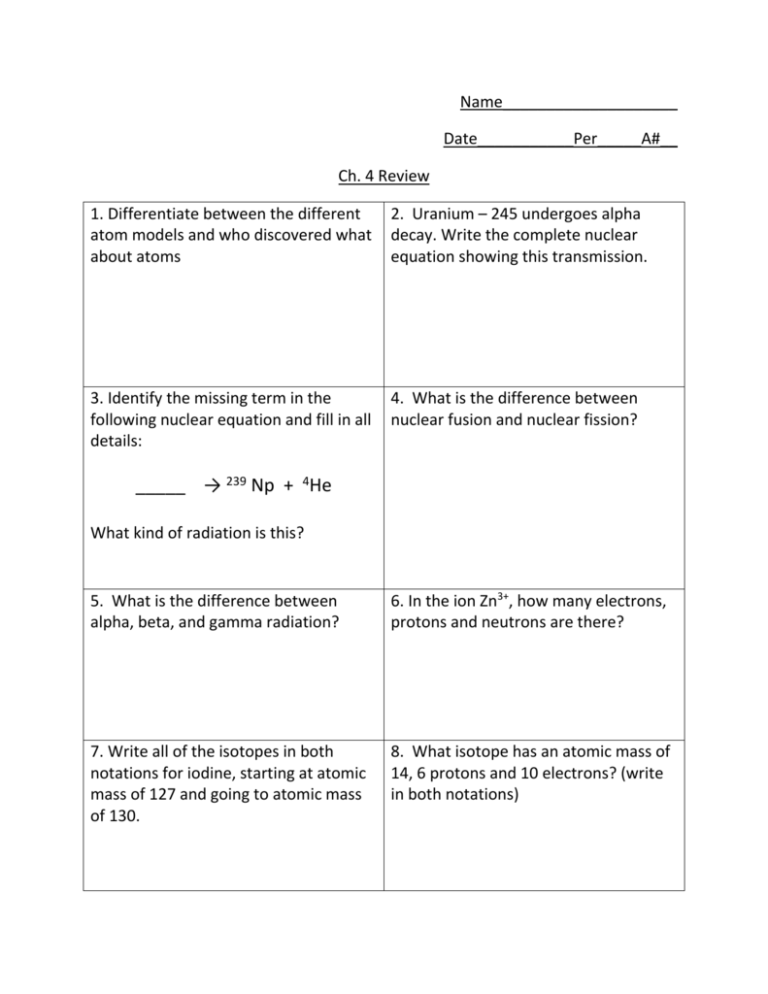
Name____________________ Date___________Per_____A#__ Ch. 4 Review 1. Differentiate between the different atom models and who discovered what about atoms 2. Uranium – 245 undergoes alpha decay. Write the complete nuclear equation showing this transmission. 3. Identify the missing term in the following nuclear equation and fill in all details: 4. What is the difference between nuclear fusion and nuclear fission? _____ → 239 Np + 4He What kind of radiation is this? 5. What is the difference between alpha, beta, and gamma radiation? 6. In the ion Zn3+, how many electrons, protons and neutrons are there? 7. Write all of the isotopes in both notations for iodine, starting at atomic mass of 127 and going to atomic mass of 130. 8. What isotope has an atomic mass of 14, 6 protons and 10 electrons? (write in both notations) 9. Write your own nuclear equation for each type of radiation. 10. Nitrogen has two naturally occurring isotopes. Nitrogen-14 has an abundance of 51.82% and a mass of 14.007 amu. Nitrogen-16 has a relative abundance of 48.18% and a mass of 16.230 amu. Calculate the average atomic mass of nitrogen. 11. Iodine has three naturally occurring isotopes, I-126, I-127 and I-128. Iodine127 has an abundance of 15.8% and mass of 127.236amu. Iodine-128 has an abundance of 4.00% and a mass of 128.362 amu. Iodine-126 has an abundance of 80.2% and mass of 126.774amu. Calculate the atomic mass of Iodine. 12. Barium-140 undergoes beta decay. Write the complete nuclear equation showing this transmission 13. Create your own problem for an ion and determining number of subatomic particles. 140 Ba → ________ + ___________