File
advertisement

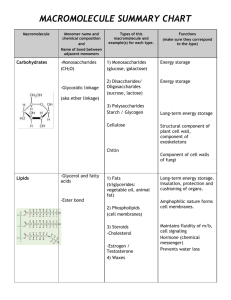
Name: __________________________________________ Date: _____ Pd: _____ Chapter 3 Review!!! 3.1 CARBON CONTAINING COMOUNDS 1. Carbon has 4 valence electrons and can make 4 bonds. 2. A single bond has 2 electrons. 3. A double bond has 4 electrons. 4. A triple bond has 6 electrons. 5. Why is carbon such a versatile molecule? (You can talk about the kinds of bonds, shapes and molecules it makes.) Carbon can make single, double or triple bonds. It can also make linear, branched and ringed structures. Carbon is essential to building large macromolecules (polymers). The macromolecules we talked about that are all composed of carbon (and other elements) are: carbohydrates, proteins, lipids and nucleic acids. 6. What is the difference between an organic and inorganic molecule? Is water organic or inorganic? Is carbon dioxide (CO2) organic or inorganic Organic molecules contain carbon. An exception to this is carbon dioxide (and carbon monoxide) since they do not contain carbon AND hydrogen. Organic compounds are also thought of as natural. Water is not organic because it contains no carbon—it’s inorganic. Inorganic compounds do not contain carbon. 7. What is a functional group? What does it do to a molecule? A functional group is a collection of atoms bound together and they change the function (reactivity) of a molecule. 8. Match the following functional groups: a. Phosphate ___iii_ i. –OH b. Hydroxyl __i___ ii. –COOH c. Carboxyl ___ii___ iii. -PO4-2 d. Amine _iv___ iv. –NH2 9. Which TWO functional groups are located on each end of an amino acid? Amine (amino) and carboxyl (carboxylic acid) 10. Which functional group is for alcohol? Hydroxyl 11. Which functional group is found in ATP? Phosphate (there are 3 of them—triphosphate) 12. What is a monomer? What is a polymer? A monomer is one unit or one molecule. Link a bunch of monomers together by a covalent bond and you get a polymer. 13. Explain what a condensation reaction is (what’s the GOAL) and water’s role in it. Condensation reaction: bind monomers together (covalent bond) and get a larger molecule, eventually building a polymer. Water is a PRODUCT in this reaction. 14. Explain what a hydrolysis reaction is (what’s the GOAL) and water’s role in it. Hydrolysis reaction: the breaking down of a polymer into its monomers by the addition of water (water is a REACTANT). 3.1 ATP (adenosine triphosphate) 1. What is the role of ATP in chemical reactions? It provides ENERGY!!! 2. How does ATP provide energy? It is a very unstable compound due to the three phosphate groups all lined up in a row (they are all negatively charged and don’t want to be next to each other—they repel each other). It takes a lot of energy to keep them there. So when one phosphate is released (via hydrolysis) it releases some of that stored energy. 3. What chemical reaction (condensation or hydrolysis) allows for ATP to release energy? Hydrolysis (add water, and break apart a piece of the molecule) 3.2 CARBOHYDRATES 1. What is a carbohydrate? Made of C, H, and O. An organic macromolecule found in biology. Most important macromolecule for energy. 2. What is the ratio of elements found in carbohydrates? 1 carbon: 2 hydrogen: 1 oxygen (example: glucose is C6H12O6) 3. What kind of energy (short or long term) do monosaccharaides and disaccharides provide? short term 4. What kind of energy (short or long term) do polysaccharides provide? long term 5. What are the monomers or building blocks of carbohydrates? saccharides (sugar) 3.2 PROTEINS 1. What is a protein? An organic macromolecule found in biology, essential for structure and control. 2. What elements are found in proteins? C, H, N and O 3. What is the monomer or building block of proteins? amino acids 4. What kind of bond holds two amino acids together? Is that a condensation or hydrolysis reaction? peptide bond, formed by a condensation reaction (you are putting two things together—amino acids, making polypeptides which eventually make up a protein) 5. How are proteins affected by changes in conditions, such as pH, temperature, or solvent? they change shape, sometimes they totally unfold (denature) 6. Enzymes help control reactions in an organism by speeding them up. What is an active site and what does that have to do with a substrate? The active site is where the substrate (reactant) binds to the enzyme. It is located on the enzyme. When the substrate binds, the reaction is sped up and a product is formed. The enzyme is like a lock and the substrate is like the key—must be an exact fit. 7. What does it mean for an enzyme to denature? Will it still react with a substrate after denaturing? Explain. Denaturing means to unfold, which totally ruins the enzyme in terms of reactivity. It will NOT react because the substrate can no longer bind to the active site if the active site is gone. 3.2 LIPIDS 1. What is a lipid? An organic macromolecule that is very nonpolar (overall). 2. Will lipids dissolve in water? Explain. NO because lipids are overall nonpolar and water is extremely polar. 3. What is a fatty acid? What are the two parts of the structure? Fatty acids make up most lipids. They have a head (carboxyl group) and tail (carbon chain, really it’s a carbon and hydrogen chain) 4. Triglycerides are made of 3 fatty acid(s) and 1 glycerol. 5. Phospholipids are made of 2 fatty acid(s) and 1 glycerol and a phosphate. 6. Waxes are made of fatty acids and long chain of alcohols. 7. What’s a phospholipid bilayer and explain its structure. It makes up a cell membrane, so it’s structural. The reason why it forms into a structure or wall is due to the nature of the head (phosphate = hydrophilic) and tail (carbon chain = hydrophobic). There are two layers and the heads of both those layers face out—one side into the cell and the other side outside the cell. The tails face inwardly in the bilayer because outside the wall is water (both inside and outside of cell). The tails HATE water and hide from it. 3.2 NUCLEIC ACIDS 1. What’s a nucleic acid? An organic macromolecule that is used to store information. The monomer is a nucleotide. 2. What’s the function of nucleic acids? Storing information (DNA and RNA) as well as storing some energy (ATP). 3. What three important structures are made of nucleic acids? What does each do? DNA = hereditary information RNA = protein synthesis, DNA synthesis, some enzymes ATP = energy VOCABULARY: You should know what each of these words means in order to understand the content of the chapter. Hydrogen bond Adhesion Cohesion Polarity (polar, nonpolar) Monomer Polymer Protein Active Site Nucleic Acid ATP Carbohydrate Monosaccharide Polysaccharide Lipid Enzyme Substrate Good luck studying! Triglyceride Phospholipid Wax Steroid Hormone Fatty Acid Reactant Product