CHEMISTRY SEPTEMBER 11, 2014
advertisement

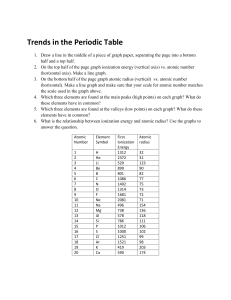
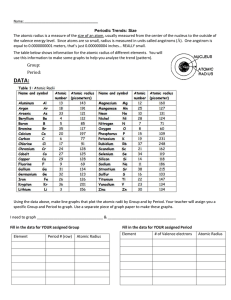
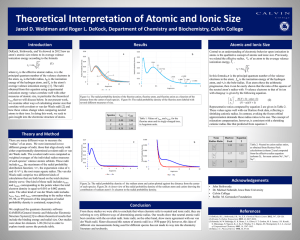
CHEMISTRY December 4, 2014 LAB 07 – GRAPHING TRENDS OF PERIODIC TABLE PRE-LAB STARTER • Work on the Pre-Lab Starter • You are seated and silent • You have 2 minutes EXPLORATORY ACTIVITY • Turn to Page 2 • You will 20 minutes to work on the Exploratory activity (the graphs are the last 2 pages of the package) • You will also need to complete questions 1-3 and 5-6 on pages 3 & 4 DEBRIEF • Given the element Phosphorous (P). Without looking at Table S, compare the atomic radius of chlorine (Cl) and Magnesium (Mg) against P. – You have 2 minutes to work with your partner to agree upon an answer. Make sure you can support your claim. – Group Share ATOMIC RADIUS The Atomic Radius is an estimate of the size of an atom or the distance from the center of the nucleus to the edge of the atom. It is an estimate due to the fact the outer edge of an atom is not distinct. Atomic radii are measured in picometers. FIRST IONIZATION ENERGY The First Ionization Energy is defined as the energy required to remove the most loosely bound (outermost) electrons from an atom. This electron is one of the valence electrons. It is measured in kilojoules/moles (kj/mol) of atoms. BOHR MODEL EXAMPLE 1 • Na and Mg BOHR MODEL EXAMPLE 2 • Na and K GUIDED PRACTICE • Map the Halogen Groups onto Graph 2 and Graph 4 • Go to Pages 3 and 4 and respond to Questions 4 and 7 EXIT TICKET • USE THE FOLLOWING 6 TERMS IN 1-2 SUPER SENTENCES – ATOMIC NUMBER, – ELEMENTS – ATOMIC RADIUS – IONIZATION ENERGY – PERIODS – GROUPS