kostner - Philippine Heart Association
advertisement

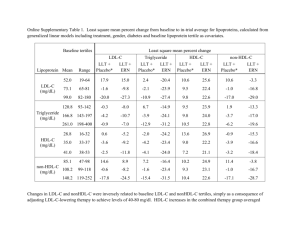
New Advances in CV Risk Reduction in High Risk Patients A/Prof. Karam Kostner Mater Hospital and University of Queensland Brisbane, Australia LDL-C Lowering HDL-C Raising TG Lowering Statins ++++ ++ ++ Niacins ++ ++++ +++ Resins ++ + 0/- Fibrates +/- +++ ++++ Ezetimibe ++ + + 0/- + ++++ LL-Therapy n-3 Ethyl Esters + = positive effect - = negative effect 0 = no effect Effect of Statins on CHD Events by LDL-C achieved: ‘ the lower the better ’ 30 4S - Placebo CHD Event Rate (%) 25 Rx - Statin therapy PRA – pravastatin ATV - atorvastatin Secondary Prevention 4S - Rx 20 TNT Diab ATV 10 15 10 LIPID - Placebo HPS - Placebo TNT Diab ATV 80 CARE - Placebo LIPID - Rx CARE - Rx Primary Prevention HPS - Placebo HPS - Rx TNT – ATV10 PROVE-IT - PRA Cards - Placebo WOSCOPS – Placebo TNT – ATV80 HPS - Rx PROVE-IT – ATV AFCAPS - Placebo 6 5 Cards - Rx AFCAPS - Rx WOSCOPS - Rx ASCOT - Placebo ASCOT - Rx 0 1.0 1.6 2.1 2.6 3.1 3.6 4.1 4.7 LDL- Cholesterol achieved, mmol/L Adapted from Rosensen RS. Exp Opin Emerg Drugs 2004;9(2):269-279 LaRosa JC et al. N Engl J Med 2005;352:1425-1435 5.2 Persistent Lipid Abnormalities in Patients on Statins: DYSIS CHD or Risk Equivalent Two or More Risk Factors Zero or One Risk Factor (n = 13,503) (n = 3,522) (11.3%) (n = 2,171) (18.3%) (70.3%) LDL-C not at goal (%) 43.4 35.7 16.7 Non–HDL-C not at goal (%)* 71.1 56.8 35.8 Low HDL-C (%) 35.9 33.6 1.4 Elevated triglycerides (%) 40.9 41.5 20.7 High triglycerides (%)** 67.4 70.2 69.5 Alexander W. American college of cardiology, 58th annual scientific session. P T. 2009;34(5):258-260 PROVE-IT: Residual CVS Events Risk pravastatin 40 mg LDL-C reduction 10% Residual Risk atorvastatin 80 mg LDL-C reduction 42% Cannon CP et al. N Engl J Med 2004; 350: 1495–1504 16% reduction p=0.005 Approaches to Reduce Residual Risk Low Density Lipoprotein (LDL) Triglyceride High Density Lipoprotein (HDL) ApoA-1 Lp(a) Inflammation BP, Smoking, Weight, Stress, Pollution What is the LDL-cholesterol level we should aim for? CARDS-P CARDS-S “in primary prevention the event rate is predicted to approach zero at LDL of 57 mg/dl = 1.5 mmol/L” IMPROVE-IT (Ezetimibe +Sim 40) “in secondary prevention the event rate is predicted to approach zero at LDL of 30 mg/dl = 0.8 mmol/L” O’Keefe, JACC 2004; 2142-6 “The Rule of 6” for Statins Effect of Statin Therapy on LDL-C Levels: “The Rule of 6” -6% Statin 10 mg 0 10 20 30 -6% 20 mg 40 mg 40 % Reduction in LDL Cholesterol -6% 80 mg 50 THREE-STEP TITRATION 60 Ezetimibe: Efficacy Mean % Change from Baseline LDL-C Triglyceride HDL-C 5 3.5 * 0 -0.4 -0.2 -1.3 -5 -4.9 -10 -15 * P < 0.05 vs placebo -20 -18.5 * Placebo Ezetimibe 10 mg (n=123) J Am Coll Cardiol 2000; 35(supp A):257A Effect of ezetimibe coadministered with statins in heterozygous FH patients Piciotta L et al. Atherosclerosis 2006 SHARP: Major Atherosclerotic Events Event Eze/simv (n=4650) Placebo (n=4620) Major coronary event Non-haemorrhagic stroke Any revascularization 213 (4.6%) 230 131 (2.8%) 174 284 (6.1%) 352 Major atherosclerotic event 526 (11.3%) 619 (13.4%) Other cardiac death Haemorrhaghic stroke 162 (3.5%) 182 45 (1.0%) 37 Risk ratio & 95% CI (5.0%) (3.8%) (7.6%) 16.5% SE 5.4 reduction (p=0.0022) (3.9%) (0.8%) Other major vascular events 207 (4.5%) 218 (4.7%) 5.4% SE 9.4 reduction (p=0.57) Major vascular event 15.3% SE 4.7 reduction (p=0.0012) 701 (15.1%) 814 (17.6%) 0.6 0.8 Eze/simv better 1.0 1.2 1.4 Placebo better Risk Factor Components of the Atherogenic Lipid Profile LDL-C Lp(a) HDL-C Accelerated Atherosclerosis + Cardiovascular Disease Triglycerides TG-rich Lipoproteins Fasting Nonfasting Remnant Lipoproteins Chylomicrons VLDL Treatment of elevated triglycerides 1) 2) 3) 4) 5) achieve LDL goal low fat diets,weight red. and physical activity fibrates, nicotinic acid high dose fish oil improve diabetic control Reasons for Combination Therapy To achieve LDL-C goal when monotherapy inadequate (elevated LDL-C) To achieve Non- HDL-C or apoB, Lp(a) goal after LDL-C goal achieved (mixed HL) To reduce Triglycerides in severe hypertriglyceridaemia (TG > 5 mmol/L) Common Combination Therapies in Lipid Lowering “Safer” combinations • Statins + BABRs for LDL-C lowering • Statins + ezetemibe for LDL-C lowering • Statins + niacin for LDL-C and TG lowering • Statins + omega-3 ethyl esters for TG lowering Combinations that may require additional monitoring • Statin + fibric acid for combined dyslipidemias • Statin + fibric acid + niacin + for combined dyslipidemias Ezetimibe/Simvastatin (VYTORIN) Significantly Reduces LDL-C Across the Dose Range Compared with ROSUVASTATIN Ezetimibe/simvastatin Rosuvastatin 10/20 mg (n=476) 10 mg (n=475) 10/40 mg 20 mg (n=477) (n=478) 10/80 mg (n=474) 40 mg (n=475) Mean % change from baseline to week 6 0 –45 –45.8% –50 –51.5%a –52.3% –55 –54.8%b –56.7% –60 –61%a –65 aP<0.001; bP=0.001 vs rosuvastatin Adapted from Catapano AL et al Curr Med Res Opin. 2006;22:2041–2053. SAFARI Trial: Combination Therapy with Simvastatin and Fenofibrate in Patients With Combined Hyperlipidemia * * * * N=618 *P<.001 versus simvastatin Grundy SM et al Am J Cardiol 2005;95:462-468. Efficacy of Fixed-Dose Niacin ER/Simvastatin Combination Therapy SEACOAST I Median % Change From Baselinea 30 ** 24.9 Simvastatin 20 mg (n = 90) ** 20 Niacin ER/Simvastatin 1000/20 mg (n = 78) Niacin ER/Simvastatin 2000/20 mg (n = 40) 10 6.7 18.3 0 -10 -7.1 -7.4 -7.6 -13.1-14.2 -13.9 -15.3 -20 * -30 -40 -22.5 * -26.5 ** 163.5 156.3 -25.0 * Non–HDL-C Median Baseline, 155.0 mg/dL -16.7 HDL-C LDL-C 115.0 119.0 112.8 43.0 42.5 42.8 Lp(a) TG ** -38.0 196.5 194.5 ** 212.3 17.0 12.0 10.0 aSimvastatin 20 mg baseline *P<.01 versus simvastatin 20 mg; **P<.001 versus simvastatin 20 mg Ballantyne C et al Am J Cardiol 2008;101:1428 Efficacy of Extended-Release Niacin HDL-C Change from Baseline 30 20 10 10% 0 –3% -10 -20 –5% -30 22% –9% –14% –17% –12% –22% –17% –11% –24% –28% -40 -50 15% 500 mg 26% 30% 29.5% –21% LDL-C Lp(a) –30% –26% –35% –44% TG –39% 1000 1500 2000 2500 3000 mg mg mg mg mg Goldberg A et al Am J Cardiol 2000;85:1100-1105. Use of Niacin 1) High risk (post MI, ACS, DM) Addition to statin in patients with low HDL 2) Combined Hyperlipidemia (elevated TG and LDL) 3) Elevated Lp(a) Other Uses: Statin intolerant patients and FH Slide Source Lipids Online Slide Library www.lipidsonline.org Kostner, 2011 Most Patients on ER Niacin Therapy Do Not Reach Therapeutic 2g Dose 100% Percent Users 80% >1500 mg 1001-1500 mg 751-1000 mg 501-750 mg <=500 mg 60% 40% 20% 0% 4 wk 8 wk N=14,386 N=6,349 12 wk 24 wk 1y N=5,277 N=5,402 N=2,104 Retrospective cohort study using administrative claims data from 2000 to 2003 Ingenix Lab/Rx Database™. Kamal-Bahl et al. Dosage and Titration Patterns of Extended Release Niacin in Clinical Practice. Abstract presented at AHA 7th Scientific Forum on Quality of Care and Outcomes Research in Cardiovascular Disease and Stroke, Washington, D.C., May, 2006. Asia Flushing Study (PN 056): Results Percent of Patients 100 80 60 40 Maximum GFSS, Presented as Percent of Patients 1.5% 6% 8.5% 12% Extreme (GFSS 10) 18% 14% Severe (GFSS 7-9) Moderate (GFSS 4-6) 26% None/Mild (GFSS 0-3) 88% 76% 50% 20 0 PBO (n=66) ERN/LRPT (n=130) N-ER (n=134) • ERN/LRPT patients had significantly (p<0.001) less flushing vs. N-ER patients, as measured by maximum GFSS categorized as none/mild, moderate, severe, or extreme. • Significantly fewer ERN/LRPT vs. N-ER patients had: – Moderate or greater flushing: 24% vs. 50% (p<0.001) – Severe or greater flushing: 10% vs. 24% (p=0.003) – Discontinuation due to flushing: 0.8% vs. 3.7% (p<0.001) Debra Kush et al. Cardiology 2009;114:192–198 Summary of Adverse Experiences (AEs) Incidence of treatment-relateda AEs was similar between ERN/LRPT and ERN or NSP Study Parameter Treatment-related clinical AE Treatment-related serious clinical AE Discontinued due to treatment-related clinical AEb ERN/LRPT N=2,548 ERN or NSP N=1,268 Simvastatin or Placebo N=931 n % n % n % 901 35.4c,d 501 39.5 156 16.8 8 0.3e,f 1 0.1 1 0.1 328 12.9 204 16.1 28 3.0 The 2 primary reasons for discontinuation: Flushing symptoms: ERN/LRPT 7.2%; ERN or NSP 16.6% Clinical AEs: ERN/LRPT 9.7%; ERN or NSP 7.0% aDetermined by the investigator to be possibly, probably, or definitely treatment-related. patients discontinued due to flushing without an associated AE report. c95% CI for difference with ERN or NSP does not include 0. d95% CI for difference with simvastatin or placebo does not include 0. e95% CI for difference with ERN or NSP includes 0. f95% CI for difference with simvastatin or placebo includes 0. bSome Paolini et al. Cardiol Clin. 2008;26:547–560. Dose Range of Omega-3 Required To Reduce Triglycerides 4 capsules of Omacor® to reduce triglycerides TG 1.8-5.0 mmol/L at baseline (after 8-week run-in) 27 Median Change From Baseline (%) Combination Omega-3 and Simvastatin (COMBOS) in Patients with HyperTG 5 Non-HDL-C TG VLDL-C 0 –5 –10 0.7‡ –2.2 –9.0* HDL-C 3.4* –2.8 –6.3 Apo B –1.2 –1.9 –4.2† –7.2 Additional changes to baseline simvastatin therapy –15 –20 –25 –30 LDL-C Omaacor 4 g/d + simvastatin 40 mg/d –29.5* –27.5* Placebo + simvastatin 40 mg/d *P <0.0001 between groups †P = 0.0232 between groups ‡P = 0.0522 between groups Davidson MH et al. Clin Ther. 2007;29(7):1354-1367. Lp(a): Epidemiology, Pathophysiology and Therapeutic Considerations Risk of Myocardial Infarction by Extreme Levels of Lipoprotein(a) in the General Population Kamstrup, P. R. et al. JAMA 2009;301:2331-2339 For any given RF, LP(a) augments risk.. ATHEROGENICITY of Lp(a): MECHANISMS Treatment of elevated Lp(a) 1) 2) 3) 4) 5) achieve LDL goal ACE in proteinuric patients Nicotinic acid LDL-apheresis Anti Lp(a) antisense and other novel therapies Aggressive LDL und Lp(a) Reduction in FH Patients Hoffmann U, Kostner K et al. Am J Cardiol. 2003 Feb 15;91(4):461-4 3000 2500 Calcified Plaque Volume in mm3 80 60 change (%) 40 20 0 -20 -40 2000 1500 1000 -60 -80 TC TG LDL Lp (a) HDL 500 -100 0 Baseline 1 Follow-up 2 Efficacy of Extended-Release Niacin HDL-C Change from Baseline 30 20 10 10% 0 –3% -10 -20 –5% 15% 22% –9% –14% –17% –12% –22% –17% –11% –24% -30 –28% -40 -50 500 mg 26% 30% 29.5% 1000 mg 1500 mg Goldberg A et al. Am J Cardiol 2000;85:1100-1105. –21% LDL-C Lp(a) –30% –26% –35% –44% –39% TG 2000 mg 2500 mg 3000 mg Inverse Relationship between Bile Acids and Plasma Lp(a) 20 patients with obstructive cholestasis before and after surgery 200 70 60 150 100 Lp(a) in mg/dl Total bile acids µmol/L 50 Mean ± SEM 98.9 ± 9.2 40 Mean ± SEM 20.3 ± 4.4 30 20 50 10 Mean ± SEM 2.7 ± 1.1 Mean ± SEM 7.1 ± 0.6 0 Before therapy After therapy 0 Before therapy After therapy Bile acids are ligands for FXR 0.2% cholic acid treatment reduces plasma apo(a) concentration in wt – mice but not in FXR-/- mice 100 50 0 ko -C A A -C wt -C on tro l 0 ns l * 50 150 ko -c on tro 100 Double tg apo(a)-YAC X FXR-/apo(a) levels in plasma (% ) 150 wt apo(a) levels in plasma (%) Single tg apo(a)-YAC Plasma apo(a) levels were measured using DELFIA I. Chennamsetty, T. Claudel, K.Kostner et al. J Clin Invest.doi:10.1172/JCI45277;2011 The selective FXR ligand GW4064 decreases apo(a) gene expression in YAC- apo(a) Tg mice (n=3) i.p. injection of the FXR ligand GW4064 20 15 10 ** 5 0 G 1.5 1.0 *** 0.5 0.0 g/ kg 30 m 40 6 W G I. Chennamsetty, T. Claudel, K.Kostner et al. J Clin Invest.doi:10.1172/JCI45277;2011 4 V Quantifying hepatic apo(a) gene and protein expression q-PCR eh ic le 16hr-harvesting the tissues apo(a) mRNA levels / cyclophilin V W 40 6 4 eh ic le (30mg/kg body wt) (n=3) apo(a) levels in plasma mg/dL ELISA Selected Pipeline Therapies Therapies to decrease LDL particle production Therapies to increase LDL particle clearance Microsomal triglyceride transfer protein (MTP) inhibitors Apolippoprotein B antisense Therapies to increase HDL CETP Inhibitors Stein EA. Endocrinol Metab Clin North Am 2009;38:99-119. Davidson MH. Curr Atheroscler Rep 2008;11:67-70. Thyroid hormone analogue Proprotein convertase subtilisin/kexin type 9 inhibitor Squalene synthase inhibitors Apolipoprotein B-100 Antisense Oligonucleotide (ASO) Therapy: Mipomersen Mipomersen: second generation ASO that inhibits apolipoprotein B-100 protein synthesis1 Phase 2 studies2,3 in patients on statins and other lipidlowering agents showed mipomersen dose-dependently reduced: ‘ Shooting the Messenger ’ Apo B LDL-C Non-HDL-C Triglycerides (TGs) 1. Crooke R, et al. In: Crooke ST, ed. Antisense drug technology: principles, strategies and applications. 2nd ed. Boca Raton, Florida: CRC Press, 2007:601-639. 2. Kastelein JJ, et al. Circulation. 2006;114(16):1729-1735. 3. Stein EA. Endocrin Metab Clin N Am. 2009; 38:99-119. Results – LDL Cholesterol Mean LDL-C change from baseline to PET Mipomersen: 11.4 mmol/L to 8.4 mmol/L (mean reduction 24.7%) Placebo: 10.4 mmol/L to 10.1 mmol/L (mean reduction 3.3%) Raal FJ et al Lancet 2010;375:998-1006. Results – Lipoprotein (a) Mean LDL-C change from baseline to PET Mipomersen: 0.6 g/L to 0.4 g/L (mean reduction 31.1%) Placebo: 0.7 mmol/L to 0.6 mmol/L (mean reduction 7.9%) Raal FJ et al. Lancet 2010;375:998-1006. Proprotein Convertase Subtilisin/Kexin Type 9 Member of family of proteases that degrade LDL-Receptor Mutations leading to loss of function are associated with lifelong low LDL-C levels and decreased risk of cardiovascular disease Inhibitors of PCSK9 are in development Stein EA Endocrinol Metab Clin North Am 2009;38:99-119. Horton JD, Cohen JC, Hobbs HH J Lipid Res 2009; 50: S172-177 Cholesterol Ester Transfer Protein (CETP) Lipoprotein Binding Surface Effects and Safety of Anacetrapib Bloomfield D et al Am Heart J 2009;157:352-60.e2 Cannon CP et al N Eng J Med 2010, November 17 on-line Results: HDL-cholesterol Bloomfield D et al. Am Heart J 2009;157:352-60.e2 Results: LDL-cholesterol Bloomfield D et al Am Heart J 2009;157:352-60.e2 Results: Lp(a) Bloomfield D et al. Am Heart J 2009;157:352-60.e2 Conclusions Extensive evidence suggests statins as initial therapy for dyslipidemia ( FH, DM, CHD), except in severe hyperTG Consider adding second or third agent when LDL-C (ezetimibe) or non-HDL-C goal (niacin, fenofibrate) not achieved For high risk patients with elevated TG and/or low HDL-C, consider adding a fibrate, niacin or n-3 acid ethyl esters to LDL-C lowering therapy Combination therapy holds great promise for reducing residual CVD risk, especially with new agents in pipeline