File
advertisement
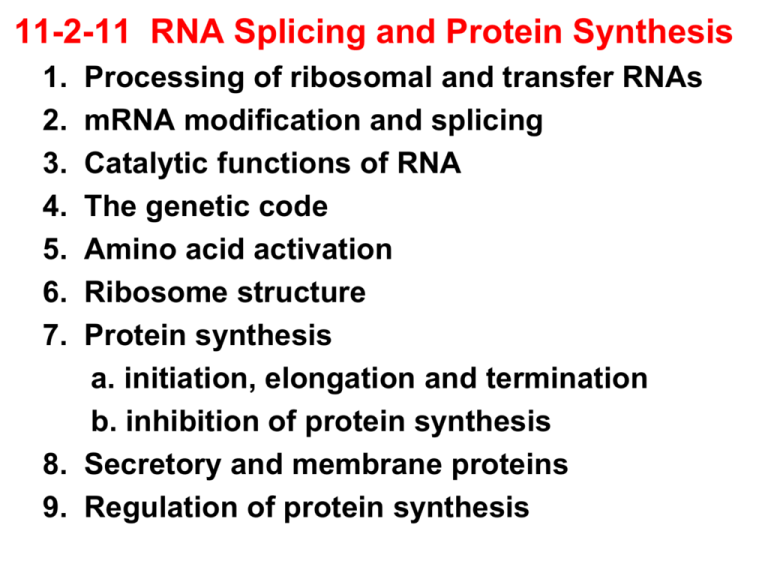
11-2-11 RNA Splicing and Protein Synthesis 1. 2. 3. 4. 5. 6. 7. Processing of ribosomal and transfer RNAs mRNA modification and splicing Catalytic functions of RNA The genetic code Amino acid activation Ribosome structure Protein synthesis a. initiation, elongation and termination b. inhibition of protein synthesis 8. Secretory and membrane proteins 9. Regulation of protein synthesis Virtually all initial transcription products are processed in eukaryotes Eukaryotic ribosomal RNAs are generated by cleavage of a precursor molecule Nucleolar RNA polymerase I transcribes a single 45S precursor containing 18S, 28S and 5.8S rRNAs 18S rRNA – component of small 40S subunit 28S, 5.8S rRNAs – components of large 60S subunit The 5S component of the 60S subunit is transcribed by RNA polymerase III Nucleotides in the pre-rRNA are extensively modified prior to cleavage Modification of pre-rRNA bases and ribose is catalyzed by snoRNPs (small nucleolar ribonucleoproteins) consisting of snoRNA and several proteins Cleavage and additional modification of pre-rRNA leads to production of mature rRNAs that are assembled together with ribosomal proteins into eukaryotic ribosomes Virtually all steps occur in the nucleolus RNA Polymerase III transcribes Transfer RNAs that are then extensively processed 5’ nucleotides (the 5’ leader) are cleaved by RNase P CCA, the amino acid attachment site, is added to the 3’ end by CCA adding enzyme tRNA bases and riboses are extensively modified Some pre-tRNAs contain introns that must be removed by splicing by endonuclease and ligase Messenger RNAs are modified and spliced RNA polymerase II transcription products are extensively modified The 5’ end of pre-mRNA is modified by addition of a 5’-5’ cap consisting of 7-methylguanylate (cap 0) Adjacent ribose residues may be methylated to form cap 1 or cap 2 5’ Caps stabilize mRNAs and enhance translation Most pre-mRNA 3’ ends are modified by polyadenylation to create poly(A) tails 3’ nucleotides are removed from the primary transcript before addition of poly (A) An internal AAUAAA sequence in the primary transcript is recognized by a specific endonuclease that removes downstream nt’s Poly(A) polymerase then adds about 250 adenylate residues to the 3’ end of the transcript Poly(A) tails stabilize the transcript and enhance translation efficiency Introns are spliced from pre-mRNAs Introns are precisely marked by splice sites Introns begin with GU and end with AG 5’ splice sites are marked by the consensus sequence AGGUAAGU in vertebrates 3’ splice sites are marked by the polypyrimidine tract (10 U or C residues) Small nuclear RNAs in spliceosomes catalyze pre-mRNA splicing snRNAs contain fewer than 300 nucleotides and some are essential to the splicing process snRNAs associate with specific proteins to form small nuclear ribonucleoprotein particles (snRNPs), or “snurps” In mammals splicing is initiated by recognition of the 5’ splice site by the U1 snRNP, which contains a 6 base pair sequence that base pairs with the 5’ splice site U1 snRNP binding initiates spliceosome assembly U2 snRNP then binds the “Branch site” Preassembled U4-U5-U6 join U1-U2 to complete spliceosome assembly Splicing begins when U5 interacts with the exon sequence in the 5’ splice site and then the 3’ exon U6 disengages from U4 and interacts with U2 and the 5’ end of the intron displacing U1 U2 and U6 thus form the catalytic center U4 is an inhibitor that masks U6 until the specific splice sites are aligned The ends of the intron are thus brought together, resulting in “transesterification” The 5’ end of the intron is cleaved to produce a lariat intermediate with the first G of the intron linked to the A in the branch region U5 holds the 3’ end of exon 1 near the 5’ end of exon 2, resulting in transesterification 2 Transesterification 2 connects exon 1 with exon 2, generating the spliced product U2, U5 and U6 bound to the excised lariat intron are released to complete the splicing reaction ATP powered RNA helicases are required to unwind RNA helices and create the alternative base pairs needed in splicing Mutations that affect Pre-mRNA splicing cause disease Mutations can be cis-acting (affecting pre-mRNA) or trans-acting (affecting splicing factors) Cis-acting mutations cause some thalassemias hereditary anemia caused by defective hemoglobin synthesis The hemoglobin b gene has 3 exons and 2 introns Cis-acting mutations can affect splice sites Splicing mutations result in incorrectly spliced mRNA that create translation stop sites preventing formation of full length hemoglobin b Mutations affecting splicing are estimated to cause at least 15% of all genetic diseases Alternative splicing yields protein diversity Different combinations of exons within the same gene may be spliced into mature RNA to produce distinct forms of the protein for specific tissues, developmental stages or signaling pathways Alternative splicing is controlled by trans-acting factors that differ in different cells Alternative splicing expands the versatility of genomes via combinatorial control In humans two different hormones are produced from a single calcitonin-CGRP pre-mRNA calcitonin-generelated protein, a vasodialator calcium and phosphate metabolism RNA can function as a catalyst - Ribozymes Splicing is mainly catalyzed by RNA molecules, with proteins playing a supporting role RNase P has an RNA component that contributes to cleaving nucleotides from the 5’ end of tRNA precursors Ribosomal RNAs are catalytic during translation Ribosomal RNA processing in Tetrahymena contains a 414 bp “self-splicing” intron