2.3H (Atomic Theory Day 2) - teacherstroh
advertisement
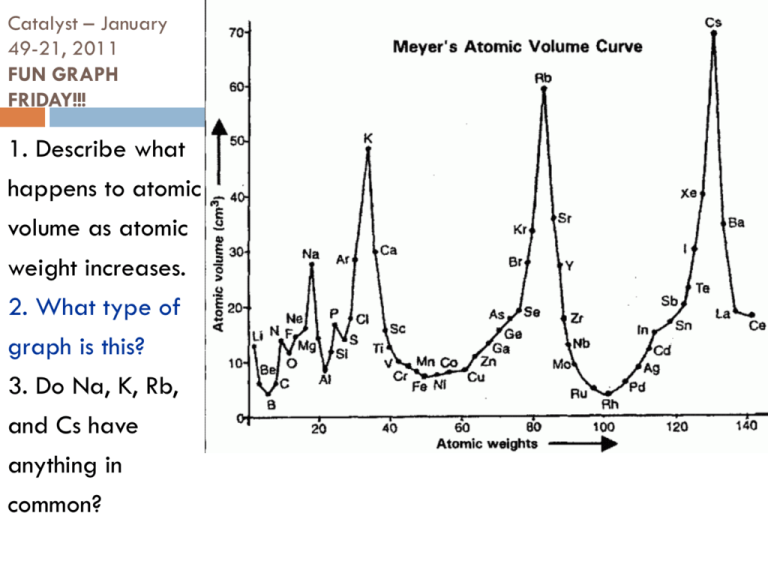
Catalyst – January 49-21, 2011 FUN GRAPH FRIDAY!!! 1. Describe what happens to atomic volume as atomic weight increases. 2. What type of graph is this? 3. Do Na, K, Rb, and Cs have anything in common? Today’s Agenda Catalyst Quick Review of Day 1! Atomic Theory Part 2 Atomic Facebook/Isotope Challenge! SCIENTIFIC SHOWDOWN: Andre vs. Jordan Group of the Week and Tests Back Exit Question HW: Unit 1 Resume Today’s Objectives SWBAT describe the evolution of atomic theory from 1898 to present. SWBAT write a conclusion and defend a theory using evidence. 2nd Period (58% of Class) Unit 1 Masters (85% or above) Bethany Alumbaugh Jordan Baye Marissa DeQueant Christopher Gordon Spencer Gros Frank Hyler Josh Jackson Tywanda Jacobs Casey Jones Richielle Kelly Karen Nguyen Baily Payne (100!) Phoebe Plaisance Kyle Robichaux Michael Tran 3rd Period (67% of Class) Unit 1 Masters (85% or above) Kat Adams (100!) Ja’Shion Alexander Paula Datri Gilberto Frutos Robert Gaines A’Lena Garrett Destiny Gibson Daniel Jackson Epiphany Johnson Julia Lewin (100!) Katelyn Lewis Kendell Nabonne Nadia Perrilliat Jill Robinson Carolina Torres Victoria Zelaya 4th Period (43% of Class) Unit 1 Masters (85% or above) Carlee Blackley Darrell Chandler Eugene Hollingshed Shannon Malonson Tarje Marks Gecoba Robinson Gino Sanchez William Smith Centoria Turner Adriona Watkins Pop Quiz #3 1. 2. 3. 4. 5. Define matter. Fill in the blank: Matter is composed of tiny particles called _________. What particle did Thomson discover? Who discovered the nucleus? Fill in the blanks: Isotopes are atoms with the same number of ________ but different numbers of ________. Quick Review Democritus – 420 B.C. – First to think atoms exist Dalton – 1808 – First REAL Atomic Theory Not a theory – no proof Based on scientific evidence JJ Thompson – 1897 – Cathode Ray Experiment Showed presence of small negative parts of atoms These parts are called electrons Implied presence of positive parts Showed atoms are NOT indivisible Developed Plum Pudding Model Ernest Rutherford Experiment: Gold Foil Experiment Where: University of Manchester (England) When: 1909 Lil’ Ernie Rutherford on the Scene! Rutherford shot positively charged alpha particles at a very thin piece of gold foil What Lil’ Ernie thought would happen… If the Plum Pudding model was true, all of the positively charged alpha particles would have gone straight through the foil What Actually Happened… Almost all of the alpha particles went straight through, but some were deflected 1 in 8000 of the particles was deflected ? Simulation http://phet.colorado.edu/simulations/si ms.php?sim=Rutherford_Scattering What do you think? Pretend you are Rutherford As Rutherford, what conclusion would you make based on the data from the Gold Foil Experiment? Hint: Positive repels positive, negative repels negative = Rutherford’s Conclusions 1.The atom is mostly empty space through which negatively charged electrons move 2.There is a tiny, dense region in the center of the atom called the nucleus (positively charged) - + - - - What’s this empty space idea? The ratio of the size of the nucleus to the diameter of the orbits of electrons can be compared with placing a marble in the middle of a football stadium! Relative Size of the Nucleus to the Atom But this wasn’t all the way right! Keeping in mind that opposites attract, What happens to the negatively charged particles that are orbiting the positively charged nucleus? The Next Great Idea In 1913, Niels Bohr considered the problem and looked at new information Scientists knew that moving electrons could create light when they released energy They noticed each element only let out certain wavelengths of light These were called emission spectra Analyzing the Spectra Bohr inferred from the spectra that the electrons could only exist certain distances from the nucleus They could jump from level to another to create light But they could not exist between levels Niels Bohr (1913) •Rutherford’s nucleus idea is good, but… •Electrons orbit around the nucleus in circular paths called energy levels! Valence electrons are electrons on the outer energy level! NOT BOHR-ING AT ALL!!! But Someone Knew This Wasn’t Right There were other emission line spectra for larger atoms that did not fit the Bohr Model… So in 1924 Erwin Schrödinger added a new detail Think of the electrons as waves instead of particles Schrodinger’s Thinking •If electrons are like waves, then we don’t ever really know where the electrons are in the atom •I will develop the idea of ATOMIC ORBITALS! Schrodinger’s Atomic Orbitals You will learn more about this later! Facebook/Isotope Challenge You have the rest of class to work on this worksheet Use the book to help you when needed (especially on the back!) Exit Question 1. 2. What was Rutherford’s contribution to atomic theory? Describe one other game-changing contribution to the atomic theory. HOMEWORK: Unit 1 Resume QUIZ NEXT TUESDAY!!!!