Chapter 1 Structure and Bonding
advertisement
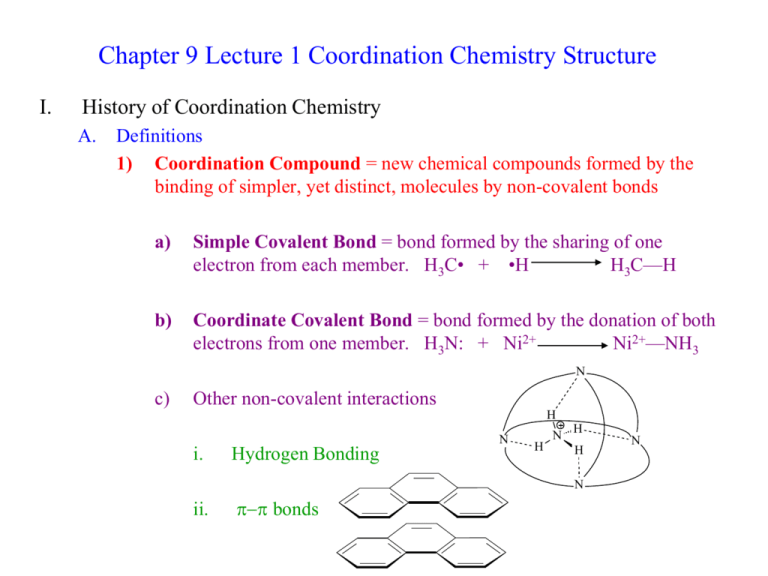
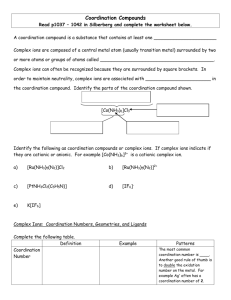
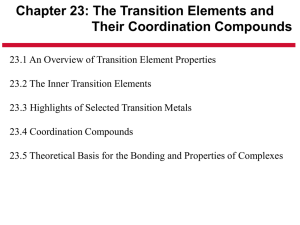
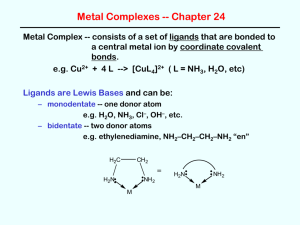
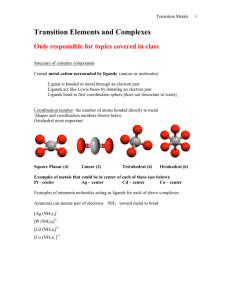
Chapter 9 Lecture 1 Coordination Chemistry Structure I. History of Coordination Chemistry A. Definitions 1) Coordination Compound = new chemical compounds formed by the binding of simpler, yet distinct, molecules by non-covalent bonds a) Simple Covalent Bond = bond formed by the sharing of one electron from each member. H3C• + •H H3C—H b) Coordinate Covalent Bond = bond formed by the donation of both electrons from one member. H3N: + Ni2+ Ni2+—NH3 N c) Other non-covalent interactions H N i. Hydrogen Bonding N H H H N ii. p-p bonds N 2) B. Ligand = atom, ion, or molecule that can donate a pair of electrons to a metal ion (A Lewis Base) = H2O, NH3, ClWerner Complexes 1) Alfred Werner (1866-1919) was the father of modern coordination chemistry 2) Early theory of coordination compound structure a) Blomstrand (1826-1894) and Jorgenson (1837-1914) b) The “valence” or charge of the metal ion determined how many bonds it could form: Co3+ could form 3 bonds c) This idea stressed the similarity to organic chemistry (C could form 4 bonds) d) NH3 could form chains, just like chains of CH2 e) Only Cl- attached to an NH3 could dissociate Evidence: -Amount of AgCl precip -Conductance Value 3) Werner’s Theory a) Metals interact with 6 ligands in octahedral geometry to form “complex ions” i. Primary or Inner Coordination Sphere = ligands with bonds to Metal ii. Secondary or Outer Coordination Sphere = more loosely bound anions present only to balance charge b) Explains multiple complexes of the same sets of ligands in diff. numbers i. [Co(NH3)6]Cl3 [Co(NH3)5Cl]Cl2 [Co(NH3)4Cl2]Cl [Co(NH3)3Cl3] ii. Different numbers of ions are produced due to outer sphere dissociation c) Explains multiple complexes with exact same formula = isomers Finally convinced Jorgenson by synthesizing both Optical Activity only possible for octahedral structure d) Further ideas of Werner proved correct as well i. His use of “inert” or slow-reacting metal ions (Co3+, Cr3+, Pt2+) let him successfully isolate all of the isomers ii. Coordination Number = the number of ligands a given metal ion prefers to have bound. Most first row transition elements prefer 6. Pt2+ prefers 4 = square planar geometry. iii. CoA4B2 only has two isomers. Trigonal Prismatic and Trigonal Antiprimatic should give 3 isomers. Octahedral only has two possible isomers. Thus the structure must be Oh. iv. PtA2B2 only has two isomers. It must be square planar rather than tetrahedral, which would only have 1 isomer. v. e) Water completes the Inner Sphere coordination in aqueous solutions: NiCl2 + H2O [Ni(H2O)6]Cl2 For his work on Coordination Chemistry, Werner was awarded the Nobel Prize in 1913 e) I. Problem with Werner’s Theory: disobeys octet rule. Solution: use d-orbitals so it becomes an 18-electron rule. [Co(NH3)6]3+ has 6 d e- + 12 e- from NH3 1s + 3p + 5d orbitals x 2 e- each = 18 total electrons possible Nomenclature of Coordination Complexes A. Ligands 1) Ligands are often organic molecules with IUPAC names 2) Common names are most often used for the ligands in coordination compounds 3) Chelate = Greek for Claw = a ligand that can bind a metal ion by more than one point of attachment. a) Bidentate = “two teeth” = NH2CH2CH2NH2 = ethylenediamene = en b) Tridentate = NH2CH2CH2NHCH2CH2NH2 = diethylenetriamine = trien c) Tetradentate = cyclam 4) Table 9-2 lists common ligands, names, structures, and abbreviations B. Naming and Writing Formulas of Coordination Compounds 1) The cation comes first, then the anion(s) a) diamminesilver(I) chloride [Ag(NH3)2]Cl b) potassium hexacyanoferrate(III) K3[Fe(CN)6] 2) Inner Sphere Complex Ion is enclosed in brackets a) Ligands are named before the metal b) Metal is written first in the formula c) A space only between cation and anion d) No capitalization is needed i. tetraamminecopper(II) sulfate [Cu(NH3)4]SO4 ii. hexaamminecobalt(III) chloride [Co(NH3)6]Cl3 3) Prefixes denote the number of each ligand type. Special prefixes and parentheses are used if the ligand already contains a prefix. 2 di bis 6 hexa hexakis 3 tri tris 7 hepta heptakis 4 tetra tetrakis 8 octa octakis 5 penta pentakis 9 nona nonakis 10 deca decakis a) b) dichlorobis(ethylenediamine)cobalt(III) fluoride [Co(en)2Cl2]F tris(bipyridine)iron(II) chloride [Fe(bipy)3]Cl2 4) Ligands are named in alphabetical order not counting prefixes. a) tetraamminedichlorocobalt(III) [Co(NH3)4Cl2]+ b) amminebromochloromethylamineplatinum(II) [Pt(NH3)BrCl(CH3NH2)] 5) Ligand name alterations: a) Anionic ligands are given an -o suffix: chloro, fluoro, oxo, sulfato b) Neutral ligands keep their name: methylamine, bipyridine c) Water becomes aqua d) NH3 becomes ammine to keep separate from alkylamines 6) Two systems for showing charge or oxidation state of metal ion a) Stock system: oxidation state in Roman Numerals in parenthesis after the metal ion name. This is the most common method. b) Ewing-Basset system: charge of the total complex ion is placed in parenthesis after the name of the metal ion. c) Both systems add –ate to the metal name if the complex ion has an overall (-) charge d) e) f) [Pt(NH3)4]2+ = tetraamineplatinum(II) or (2+) [PtCl4]2- = tetrachloroplatinate(II) or (2-) [PtCl6]2- = hexachloroplatinate(IV) or (2-) 7) Isomer designations come before the rest of the name and in italics a) cis-diamminedichloroplatinum(II) b) trans-diamminedichloroplatinum(II) 8) Bridging ligands have the prefix m [(NH3)4Co(OH)(NH2)Co(NH3)4]4+ = m-amido-m-hydroxobis(tetraaminecobalt(III)) 9) Negatively charged complexes of metals originally having Latin names: Fe = ferrate Ag = argenate Sb = stibate Au = aurate Pb = plumbate Sn = stannate Au = aurate Exception: Hg still called mercurate (Latin name: hydrargyrum)