Nomenclature of Coordination Compounds
advertisement

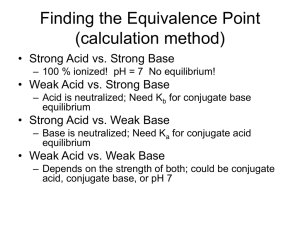
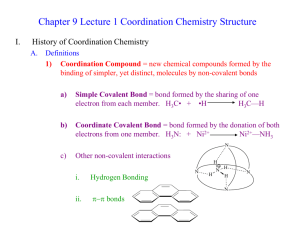
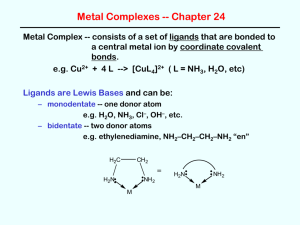
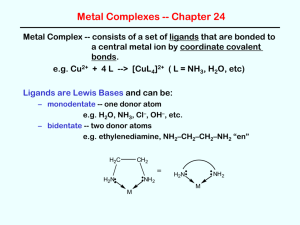
Coordination Compounds Read p1037 – 1042 in Silberberg and complete the worksheet below. A coordination compound is a substance that contains at least one ______________________ Complex ions are composed of a central metal atom (usually transition metal) surrounded by two or more atoms or groups of atoms called ________________________________________. Complex ions can often be recognized because they are surrounded by square brackets. In order to maintain neutrality, complex ions are associated with _______________________ in the coordination compound. Identify the parts of the coordination compound shown. [Co(NH3)6]Cl3 Identify the following as coordination compounds or complex ions. If complex ions indicate if they are cationic or anionic. For example [Co(NH3)6]3+ is a cationic complex ion. a) [Ru(NH3)5(N2)]Cl2 b) [Ru(NH3)5(N2)]2+ c) [PtNH3Cl2(C5H5N)] d) [IF6]- e) K[IF6] Complex Ions: Coordination Numbers, Geometries, and Ligands Complete the following table. Definition Coordination Number Example Patterns The most common coordination number is ____. Another good rule of thumb is to double the oxidation number on the metal. For example Ag+ often has a coordination number of 2. Geometry If coordination number is ____, then geometry is ___________ If coordination number is ____, then geometry is __________, or _____________ If coordination number is ____, then geometry is ___________ Ligands O is from group 16 and has a lone pair to donate Must have at least one lone pair to donate and so often come from the following families: What is meant by the terms monodentate, bidentate, and polydentate? What is water? Rules for Naming Coordination Compounds 1. __________________________________________________________________ [Co(NH3)4Cl2]Cl “cationic complex ion” chloride 2. K[IF6] potassium “anionic complex ion” __________________________________________________________________ __________________________________________________________________ [Co(NH3)4Cl2]Cl Metal ion named second 3. Ligands named first, alphabetically Neutral ligands generally _______________________________________________ Anionic ligands _______________________________________________________ Ligand Bromide, Chloride, Fluoride, Iodide Carbonate Cyanide Hydride Hydroxide Nitrite Oxalate Sulphide Thiocyanate Ammonia Carbon monoxide Ethylenediammine Methylammine Nitrogen monoxide Pyridine Water Formula or Abbreviation Br , Cl-, F-, I- Ligand name Bromo, Chloro, Fluoro, Iodo CO32CNHOHNO2- or ONOC2O42S2SCNNH3 CO NH2CH2CH2NH2 or en CH3NH2 NO C5H 5N H 2O Carbonato Cyano Hydrido Hydroxo Nitro or Nitrito Oxalato Thio Thiocyanato Ammine Carbonyl (Ethylenediammine) Methylammine Nitrosyl Pyridine Aqua - The two ligands in [Co(NH3)4Cl2]Cl would be called: 4. _________________________________________________________________ _________________________________________________________________ The prefixes bis, tris, tetrakis, pentakis, etc are used for more complicated ligands (ones that already contain di, tri etc or those that begin with a vowel except aqua and ammine). When using these prefixes the name of the ligand is usually encased in brackets. The two ethylenediammine ligands in the complex ion [Co(en)2Cl2]NO3 would be called: 5. The oxidation state of the central metal ion is given by ________________________ __________________________________________________________________ To determine the oxidation state of the central metal ion simply use the redox rules you have learned. For example the oxidation state of the metal ion in [Co(NH3)4Cl2]Cl is ____ 6. If the complex ion is an anion ____________________________________________ __________________________________________________________________ For example the name for K[Pt(NH3)Cl5] is __________________________________ Sometimes the Latin names are used to identify the metal in anionic complex ions . Iron Gold Copper Lead Silver Tin For example, the name for Na4[FeBr6] is: __________________________________ Try These: [Ni(H2O)4Cl2] ____________________________ [Co(NH3)6]Cl3 ____________________________ ___________ potassium tetrachloroplatinate(II) ___________ tetranitrosylchromium(0) K2[Ge(C2O4)3] ____________________________ [Cr(en)3]Cl3 ____________________________ ___________ potassium tetracyanoaurate(III) ___________ ammonium hexachloroplumbate(IV) [Co(NH3)5Cl]Cl2 ____________________________ K3Fe(CN)6 ____________________________ ___________ triamminebromoplatinum(II) chloride ___________ potassium hexafluorocobaltate(III) [Co(NH3)4(NO2)Cl]Cl ____________________________ [Fe(en)2(NO2)2]2SO4 ____________________________ Silberberg p1059-1060 #47, 49, 52, 55, 57, 59, 61, 63, 65, 67, 69