Synthetic Strategies toward Substituted Benzenes
advertisement

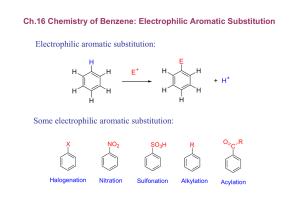
16-5 Synthetic Strategies toward Substituted Benzenes To obtain substitutions in positions incompatible with the directing sense of substituents requires a couple of synthetic “tricks”. Among these are: •Chemical interconversions of ortho, para with meta directors (nitro amino or carbonyl methylene); •Additional knowledge about practicality of certain electrophilic substitutions; •Employment of reversible blocking strategies with sulfonic acid groups (-SO3H). The sense of the directing power of substituents can be changed. The simplest way to introduce a nitrogen substituent into an arene is by nitration. The nitro group (meta-directing, deactivating) can be easily converted into the amino group (ortho, para-directing, activating) by reduction: Catalytic hydrogenation or Exposure to acid in the presence of active metals (iron or zinc amalgam) Oxidation of the amino group back to a nitro group can be accomplished using trifluoroperacetic acid. To prepare 3-bromobenzamide, direct bromination of benzamide leads to ortho and para substitution. If nitrobenzene is used instead: A similar conversion strategy can be used for alkanoyl alkyl. In the synthesis of 1-chloro-3-ethylbenzene from benzene, neither chlorobenzene nor ethylbenzene is suitable as the immediate precursor to the product; each is ortho-, paradirecting. Ethanoyl benzene is a meta director which can subsequently be reduced to the ethyl functional group. Reduction of alkanoyl- to alkylarenes provides a synthetic path that does not suffer from overalkylation or alkyl group rearrangement. Butyl benzene is best synthesized by alkanoylation with butanoyl chloride followed by Clemmensen reduction. Direct Friedel-Crafts butylation of benzene results in di- and trialkylation, as well as the formation of a rearranged product. Friedel-Crafts electrophiles do not attack strongly deactivated benzene rings. Only one of the two synthetic paths below to 1-(3-nitrophenyl)ethanone actually succeeds. The second route fails primarily due to the extreme deactivation of the nitrobenzene ring. Another factor is the low electrophilicity of the acylium ion compared to other electrophiles in aromatic electrophilic substitution. As a general rule, neither Friedel-Crafts alkylations nor alkanoylations take place with benzene derivatives strongly deactivated by meta-directing groups. Reversible sulfonation allows the efficient synthesis of orthodisubstituted benzenes. The synthesis of 1-(1,1-dimethylethyl)-2-nitrobenzene [o-(t-butyl)nitrobenzene] by direct nitration of t-butylbenzene results in very low yields. The para isomer is the dominant product in this and in most other electrophilic substitutions of an ortho, para director. The desired ortho isomer can be prepared in high yield by first sulfonating the tbutylbenzene, nitrating the p-product and then removing the sulfonate group. Because both the substituent and the electrophile are sterically bulky, sulfonation occurs almost entirely at the para position. Protection strategies moderate the activating power of amine and hydroxy groups. Electrophilic attack on benzenamine and phenol are difficult to stop at the monosubstitution stage and sometimes may involve the heteroatom rather than the aromatic ring. Protection groups, acetyl for benzenamine and methyl for phenol reduce the activating nature of the amino and hydroxy groups and protect them against reaction with the incoming electrophile. Deprotonation is by basic or acidic hydrolysis, respectively.