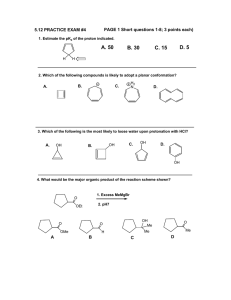
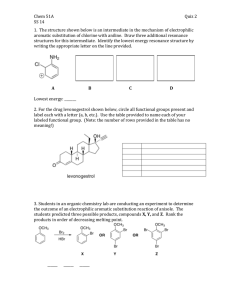
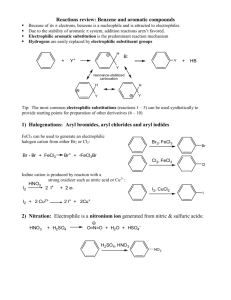
Chapter 19
advertisement
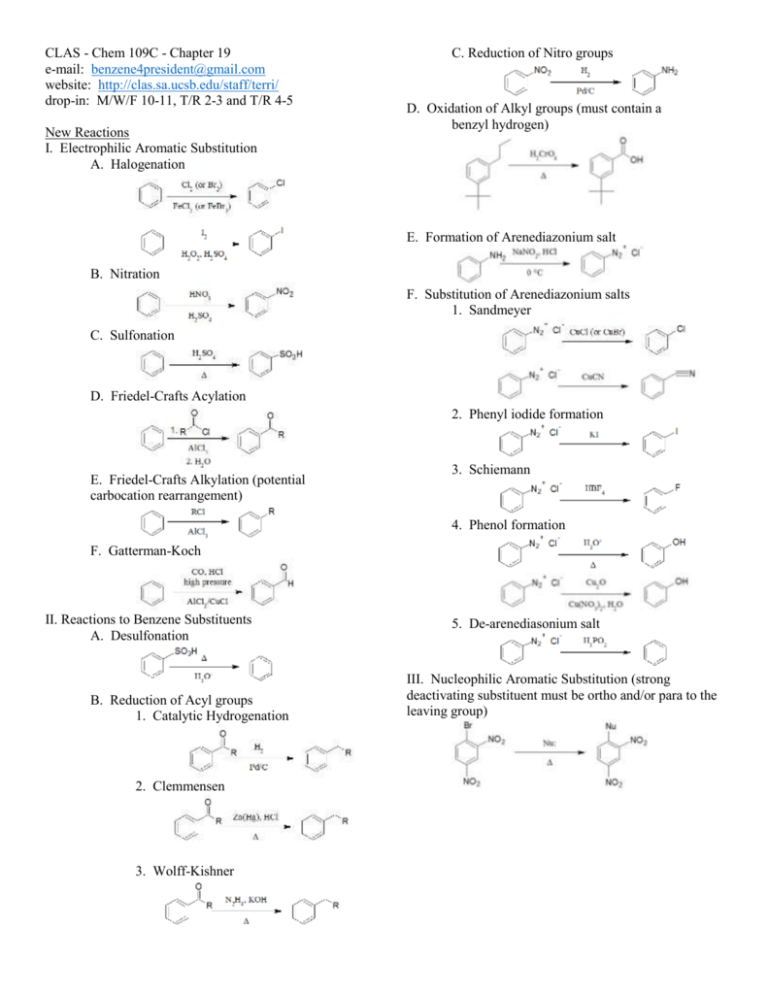
CLAS - Chem 109C - Chapter 19 e-mail: benzene4president@gmail.com website: http://clas.sa.ucsb.edu/staff/terri/ drop-in: M/W/F 10-11, T/R 2-3 and T/R 4-5 New Reactions I. Electrophilic Aromatic Substitution A. Halogenation C. Reduction of Nitro groups D. Oxidation of Alkyl groups (must contain a benzyl hydrogen) E. Formation of Arenediazonium salt B. Nitration F. Substitution of Arenediazonium salts 1. Sandmeyer C. Sulfonation D. Friedel-Crafts Acylation 2. Phenyl iodide formation E. Friedel-Crafts Alkylation (potential carbocation rearrangement) 3. Schiemann 4. Phenol formation F. Gatterman-Koch II. Reactions to Benzene Substituents A. Desulfonation B. Reduction of Acyl groups 1. Catalytic Hydrogenation 2. Clemmensen 3. Wolff-Kishner 5. De-arenediasonium salt III. Nucleophilic Aromatic Substitution (strong deactivating substituent must be ortho and/or para to the leaving group) Increasing pKa (if on a phenol) Reactivity toward Electrophilic Aromatic Substitution -NH2, -NHR, -NR2 (most reactive) -OH, -OR -NHCOR -OCOR -R -Ar, -CH=CHR Activating (more reactive than benzene) Ortho/Para Directors -H (standard) -F -Cl -Br -I -CHO -COOR -COOH -COCl -C≡N -SO3H -NH3+, -NH2R+, -NHR2+, -NR3+ -NO2 (least reactive) De-activating (less reactive than benzene) Practice Problems 1. Predict the products for the following reactions: a. g. b. h. c. i. d. e. f. j. k. Meta Directors 2. Rank the following compound in order of increasing pKa. Explain your reasoning. I. phenol II. para-methoxyphenol III. para-nitrophenol 3. Provide a possible synthesis for the following compounds starting from benzene: a. meta-nitrobenzoic acid b. para-chlorobenzoic acid c. meta-butylnitrobenzene d. para-bromostyrene e. meta-chloroaniline f. meta-bromophenol g. meta-dichlorobenzene