Accuracy & Precision (8/25) - Liberty Union High School District
advertisement
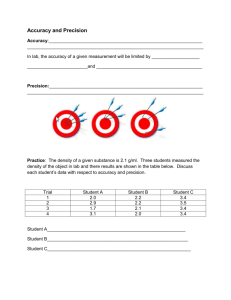
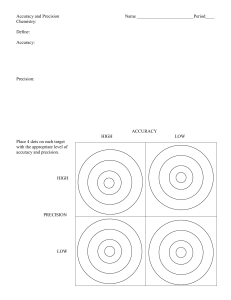
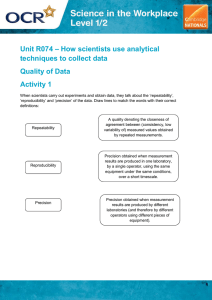
Accuracy & Precision Tuesday, August 25th, 2015 Accuracy vs Precision – Accuracy – how close a measurement is to the actual value Precision – how close a group of measurements are to each other. Look at each target below and decide whether the situation is accurate, precise, both, or neither: (note-it is “accepted” that the bull’s eye is the place everyone aims for.) Percent Error - how close a measured value is to its actual value (aka accuracy). A student takes an object with an accepted (actual) mass of 200.00 grams and masses it on his own balance. He record the mass as 196.5 g. What is his percent error? How accurate do you need to be....+/- 10% Practice Problems 1. An experimental measurement was taken of 10.4 mL and the actual measurement was 9.7 mL. What is the percent error? 2. Joe measured the volume of an object five times and got results of 34.5 mL, 34.9 mL, 34.2 mL, 33.4 mL, and 35.9 mL. The actual volume of the object was 34.1 mL. What was the percent error of his average result? 3. A student measured the temperature of boiling water and got 97.5°C. What was her percent error? Practice Problems 1. An experimental measurement was taken of 10.4 mL and the actual measurement was 9.7 mL. What is the percent error? % error = | 9.7 – 10.4 | x 100 = 7.2% 9.7 Practice Problems 2. Joe measured the volume of an object five times and got results of 34.5 mL, 34.9 mL, 34.2 mL, 33.4 mL, and 35.9 mL. The actual volume of the object was 34.1 mL. What was the percent error of his average result? 34.5 + 34.9 + 34.2 + 33.4 + 35.9 = 172.9/5 = 34.6 mL % error = | 34.1 – 34.6 | x 100 = 1.47% 34.1 Practice Problems 3. A student measured the temperature of boiling water and got 97.5°C. What was her percent error? % error = | 100°C – 97.5°C | x 100 = 2.50% 100 °C