Percent Error
advertisement

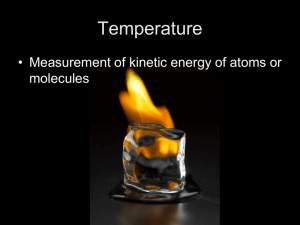
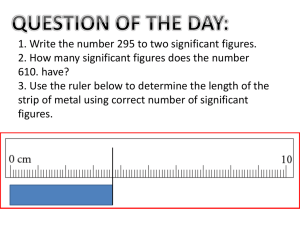
Accuracy and Precision Accuracy:_____________________________________________________________ ______________________________________________________________________ In lab, the accuracy of a given measurement will be limited by ___________________ ________________________and __________________________________________ Precision:_____________________________________________________________ ______________________________________________________________________ Practice: The density of a given substance is 2.1 g/ml. Three students measured the density of the object in lab and there results are shown in the table below. Discuss each student’s data with respect to accuracy and precision. Trial 1 2 3 4 Student A 2.0 2.9 1.7 3.1 Student B 2.2 2.2 2.1 2.0 Student C 3.4 3.5 3.4 3.4 Student A_______________________________________________________ Student B________________________________________________________ Student C_________________________________________________________ Accuracy, Precision, and Percent Error Chemistry Percent Error Percent error = Percent error will always be a ______________number. Practice: 1. The boiling point of water is 100oC. During an experiment, water came to a boil at 97oC according to the thermometer that was being used. What is the percent error of the thermometer? 2. An experiment was performed to determine the density of water. The results of the experiment showed that water had a density of 1.15 g/mL. What was the percent error in this experiment? 3. An experiment was conducted to find the mass of one mole of carbon atoms. The results of the experiment showed that a mole of carbon atoms had a mass of 15.78 g. The accepted value of a mole of carbon atoms is 16.00 grams. What is the percent error in this experiment? 4. An experiment performed to determine the density of lead yields a value of 10.95 g/cm3. The accepted value for the density of lead is 11.342 g/cm 3. Find the percent error. 5. Find the percent error in a measurement of the boiling point of bromine if the laboratory figure is 40.6oC and the accepted value is 59.35oC. 1. 3% 2. 15% 3. 1.375% 4. 3.456% 5. 31.6%