Day 1 Introduction to Chemistry and Measurement
advertisement

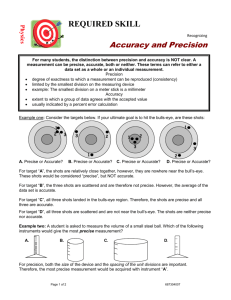
Can you hit the bull's-eye? Three targets with three arrows each to shoot. How do they compare? Both accurate and precise Precise but not accurate Neither accurate nor precise Can you define accuracy and precision? Accuracy: A measure of how close a measurement comes to the actual or true value of whatever is measured. Precision: A measure of how close a series of measurements are to one another. Three students measure the volume to be 10.2 ml, 10.3ml and 10.4 ml . • If the actual value of volume is 10.5ml • Were they precise? No • Were they accurate? No Weight Trial Result 1 2.486g 2 2.487g 3 2.487g 4 2.487g 5 2.487g Are these values precise? Yes High precision among several measurements is an indication of accuracy only if systematic error is absent . Calculates the error in a measurement Table T of Reference Tables Accepted value = the correct value based on reliable references. Experimental value = the value measured in the lab (your results). Error = accepted value – exp. value Can be positive or negative Percent Error = the absolute value of the error divided by the accepted value, then multiplied by 100% Problem: What is the percent error of a measured value of 187 lb. if the person’s actual weight is 175 lb.? Solution: 187 lb -175 lb X 100% 175 lb Percent Error = 12 X 100% 175 .0685 X100% = 6.85% Problem: Measured density from lab experiment is 1.40 g/mL. The correct density is 1.36 g/mL. • Find the percent error. From an experiment the data for the molar mass of oxygen is 30.0g. From the periodic table the mass is 32.0g. What is the percent error? 30.0g – 32.0g X 100% = 6.3% 32.0g A technician experimentally determined the boiling point for sodium (Na) to be 1105 K. Calculate the percent error. Solution = 4.4%