Charles Law Problems
advertisement

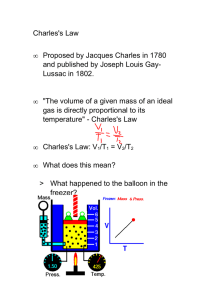
COMPLETION OF THIS PAPER IS REQUIRED. SUGGESTED DUE DATE IS: NEXT CLASS PERIOD! Name: ________________________________________________________ Date: __________ Period: _________ 11 The Behavior and Chemistry of Gases How are the properties and reactions of gases described and quantified? (CHEMACTIVITY: Charles’ Law) 2 – You Got It! 1 – Needs Some More Work! 0 – Incomplete 1. Suggested Reading… pp. 450-454 2. What two variables are related through the use of Charles Law? Describe the relationship between these two variables: V1 V2 T1 T2 3. The Charles Law formula is: Describe the variables in this formula and what this formula allows us to calculate: 4. Why is it important to use temperature expressed in Kelvin when using the Charles Law formula? Volume (L) 5. If we were to graph the relationship between temperature and volume, what would the graph look like? Temperature (K) Temperature conversions: K = °C + 273 °C = (°F-32)/1.8 6. When using Charles Law, why is it important to keep the pressure and amount of gas constant? 7. A 2.00 L sample of air is collected at 298K and then cooled to 278K. Calculate the new volume of this sample: 8. A sample of gas at 15.0°C has a volume of 2.58 L. The temperature is then increased to 38.0°C. Does the volume increase or decrease? Calculate the new volume. 9. A sample of neon gas has a volume of 250. mL at a temperature of 30.0°C. At what temperature (°C) would the volume of this sample be reduced by half? 10. A sample of helium at 27.0°C is warmed to 45.5°C. If the final volume is measure as 78 L, what was the initial volume? 11. The label on an aerosol spray can warns that the can should not be heated above 130°F. Calculate the potential volume of the gas in a 500mL aerosol can when it is heated from 25.0°C to 54.0°C. Why might this pose a problem?? Essential Content and Skills: Recognize that relationship(s) between four variables (pressure, temperature, volume, and amount) can be used to explain the behavior of gases at the molecular level How do we explain and quantify the relationship between absolute temperature (Kelvin) and volume (Charles’ Law) using mathematical formulas and graphs?