Using Charles's Law
advertisement

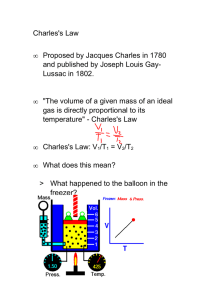
Charles's Law Lesson Objectives The student will be able to: State Charles's law. Solve problems using Charles's law. Vocabulary Charles's law: the volume of a fixed mass of gas held at a constant pressure varies directly with the absolute temperature Direct proportion: a relationship between two variables where, when one of the variables is increased, the other variable will increase by exactly the same factor The gas laws are mathematical expressions that relate the volume, pressure, temperature, and quantity of gas present. They were determined from the results of over 100 years of experimentation. They can also be derived logically by examining the present day definitions of pressure, volume, and temperature. Charles's Law The relationship between the volume and temperature of a gas was investigated by a French physicist, Jacques Charles (1746-1823). This relationship is often referred to as Charles's law. An apparatus that can be used to study the relationship between the temperature and volume of a gas is shown below. Once again, the sample of gas is trapped inside a cylinder so that no gas can get in or out. Thus, we would have a constant mass of gas inside the cylinder. In this setup, we would also place a mass on top of a movable piston to keep a constant force pushing against the gas. This guarantees that the gas pressure in the cylinder will be constant. If the pressure inside increases, the piston will be pushed up until the inside pressure becomes equal to the outside pressure. Similarly, if the inside pressure decreases, the outside pressure will push the cylinder down, decreasing the volume until the two pressures again become the same. This system guarantees constant gas pressure inside the cylinder. 1 With this set up, we can adjust the temperature and measure the volume at each temperature to produce a data table similar to the one we created for comparing pressure and volume. The picture on the left in the diagram above shows the volume of a sample of gas at , while the picture on the right shows the volume when the temperature has been raised to . After two more trials, the collected data are shown below. Charles's Law Data Trial Volume Temperature Volume/Temp 1 2 3 4 In order to find a constant from this data, it was necessary to divide each volume with the corresponding Kelvin temperature. The mathematical expression for Charles's Law is: 2 This relationship is to be expected if we recognize that we are increasing molecular collisions with the walls by raising the temperature. The only way to keep the pressure from increasing is to increase the area over which that force is exerted. This mathematical relationship is known as a direct proportion. When one variable is increased, the other variable also increases by exactly the same factor. Historical note: In addition to exploring the relationship between volume and temperature for gases, Jacques Charles was also the first person to fill a large balloon with hydrogen gas and take a solo balloon flight. Using Charles's Law Charles's law states that for a sample of gas at constant temperature, every volume divided by temperature trial will yield the same constant. Therefore, if you know the volume of a gas at one temperature, you can use this information to find either the temperature of the gas sample at another volume, or the volume of the gas at another temperature. We use the subscript 1 to represent the initial set of conditions, and the subscript 2 to represent the final set of conditions. If and then Important Note: In gas law problems, always convert temperatures to kelvin. The gas law equations do not work for temperatures expressed in the Celsius and Fahrenheit scales. Example: Calculate the decrease in temperature when 2.00 L at 20.0 °C is compressed to 1.00 L. Step 1 : Assign known values to the appropriate variable. 3 V1 = 2.00 L T1 = 20°C V2 = 1.00 L T2 = ?? Step 2: Change all temperatures to kelvin. 20°C + 273 = 293 K Step 3 : Solve the Charles's law equation for the unknown variable. Step 4 : Substitute the known values into the formula and solve for the unknown. Step 5: Change temperature values back to their original scales. 146.5 - 273 = - 107.5°C Lesson Summary Charles's law states that for a gas at constant pressure, volume in directly proportional to temperature. The volume of a mass of gas is dependent on the temperature and pressure. Therefore, these conditions must be given along with the volume of a gas. In order to receive credit for this lesson, you must click the finish button below. If you did not score an 80 or above, you will need to repeat the lesson. 4